The Shadow Pandemic: An Analysis of the Emerging Association Between Bartonellosis and Post-Acute Sequelae of SARS-CoV-2 (Long COVID)
Long COVID may mask reactivated Bartonella infections. Advanced diagnostics and targeted antibiotics could help a subset of patients misdiagnosed with post-viral illness.
Executive Summary
The global COVID-19 pandemic has left in its wake a secondary, chronic public health crisis known as Post-Acute Sequelae of SARS-CoV-2 (PASC), or Long COVID. This multisystemic condition, affecting millions worldwide, is characterized by a heterogeneous array of debilitating symptoms that persist for months or years post-infection. While research has identified several potential pathophysiological drivers—including viral persistence, immune dysregulation, and endothelial dysfunction—a definitive understanding and effective treatments remain elusive. This report synthesizes emerging evidence suggesting a clinically significant and previously underappreciated relationship between Bartonella spp. infections and Long COVID.
The central hypothesis of this analysis is that in a subset of patients, the symptom complex attributed to Long COVID may be caused or substantially exacerbated by the reactivation of a latent Bartonella infection. The profound immune dysregulation induced by SARS-CoV-2 infection appears capable of disrupting the host-pathogen balance, allowing this stealthy, intracellular bacterium to emerge from a state of dormancy. The extensive symptomatic overlap between chronic bartonellosis and Long COVID—spanning neurological, psychiatric, cardiovascular, and musculoskeletal domains—creates a formidable diagnostic challenge, potentially leading to the misattribution of a treatable bacterial illness to an intractable post-viral syndrome.
This report analyzes direct clinical evidence from case studies where Bartonella infections were "unmasked" in patients undergoing evaluation for Long COVID, often requiring advanced molecular diagnostics for identification. It explores the shared pathophysiological mechanisms, demonstrating that both SARS-CoV-2 and Bartonella target the vascular endothelium and disrupt immune function, creating a plausible basis for synergistic pathology. The profound diagnostic and therapeutic implications of this association are addressed, highlighting the limitations of conventional testing for chronic bartonellosis and the potential for targeted antimicrobial therapy to resolve symptoms in correctly identified patients. The report concludes with urgent recommendations for clinicians to consider Bartonella in the differential diagnosis of atypical or refractory Long COVID and for the research community to prioritize systematic investigation into this potential "hidden" driver of post-pandemic chronic illness.
Section 1: The Clinical Spectrum of Bartonellosis: A Persistent and Elusive Pathogen
To comprehend the potential interplay between Bartonella infection and Long COVID, it is first necessary to establish a thorough understanding of bartonellosis as a clinical entity. Far from being a simple, acute infection, bartonellosis is a group of emerging infectious diseases caused by bacteria that have evolved sophisticated mechanisms for long-term persistence within a mammalian host. This capacity for chronicity and its diverse, multisystemic clinical presentations are foundational to understanding its potential role in a complex condition like Long COVID.
1.1 Taxonomy, Species, and Transmission Vectors
Bartonellosis is the term for any infectious disease caused by bacteria of the genus Bartonella, which are Gram-negative, facultative intracellular bacteria.1 While at least 22 species have been named, a few are of primary importance in human medicine and are responsible for the majority of recognized clinical syndromes.1
The most prominent human pathogens include:
Bartonella henselae: This species is the principal causative agent of cat scratch disease (CSD), one of the most common forms of bartonellosis in the United States.4 Transmission to humans typically occurs via the scratch or bite of a cat, particularly a kitten, that is contaminated with the feces of infected cat fleas (
Ctenocephalides felis).4 Cats serve as the main reservoir, and the bacteria can be present in the blood of up to one in three healthy-appearing cats, with stray and young cats being more likely carriers.4Bartonella quintana: The etiological agent of trench fever, this species is classically transmitted by the human body louse (Pediculus humanus corporis).2 Consequently, infections are most commonly associated with populations living in crowded conditions with limited access to hygiene, such as individuals experiencing homelessness.6
Bartonella bacilliformis: This species causes Carrion's disease, a biphasic illness presenting as an acute febrile phase (Oroya fever) and a chronic eruptive phase (verruga peruana).3 Its transmission is geographically restricted to the Andes mountains of South America, where it is spread by the bite of sand flies of the genus Lutzomyia.2
While these are the classic transmission routes, emerging evidence suggests that other arthropod vectors, such as ticks, may play a role in the transmission cycle, although the U.S. Centers for Disease Control and Prevention (CDC) currently states there is no definitive evidence that ticks spread Bartonella infection to people.5 This ongoing debate highlights the evolving understanding of the epidemiology of these complex pathogens.
1.2 Pathophysiology of Persistence: Intracellular Niche and Immune Evasion Strategies
Bartonella is not merely an acute infectious agent but a master of chronic persistence. Its entire pathogenic strategy is geared toward establishing and maintaining a long-term, often subclinical, infection within the host. This biological imperative is what establishes the prerequisite for reactivation: a latent reservoir of bacteria can exist in an individual for years, potentially decades, before an immunological trigger like SARS-CoV-2 infection allows it to re-emerge.
A key feature of Bartonella infection in its natural reservoir host is a long-lasting, relapsing intraerythrocytic bacteremia, where the bacteria infect red blood cells.13 This is a crucial adaptation for ensuring efficient uptake by blood-sucking arthropod vectors.15 To achieve this, the bacteria are thought to first colonize a primary, protected niche within the host, from which they can periodically seed into the bloodstream. Endothelial cells, which line all blood vessels, are considered a principal sanctuary.3 This "hiding-seeding" mechanism allows the bacteria to evade immune clearance while maintaining the potential for transmission.20
To survive long-term within an immunologically competent host, Bartonella species have evolved a sophisticated arsenal of immune evasion strategies. These include:
Intracellular Sequestration: By invading and residing within host cells, such as endothelial cells and erythrocytes, the bacteria are shielded from antibodies and other components of the humoral immune system.18
Antigenic Variation: The bacteria can alter their surface-exposed proteins, effectively changing their appearance to evade recognition by the host's adaptive immune system.20
Immune Modulation: Bartonella can actively manipulate the host's cellular processes. For instance, B. henselae can inhibit apoptosis (programmed cell death) in infected endothelial cells, ensuring the survival of its cellular niche.14 They can also modulate cytokine production and form biofilms, further protecting them from immune attack.21
These mechanisms collectively allow Bartonella to establish a persistent, low-level infection that the host immune system contains but often fails to completely eradicate. This creates a delicate equilibrium that can be shattered by a significant physiological stressor or a secondary infection that dysregulates the immune system.
1.3 The Diverse Manifestations of Chronic Bartonellosis
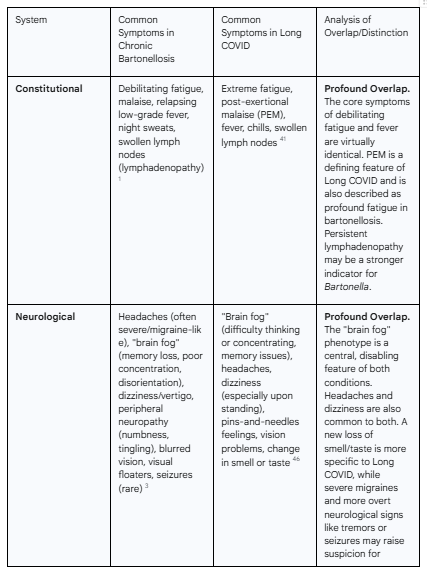
The clinical presentation of chronic bartonellosis is notoriously heterogeneous, producing a multisystemic, non-specific syndrome that strongly mimics other complex chronic illnesses, including fibromyalgia, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and, as this report will detail, Long COVID. This extensive symptomatic overlap is a central reason why the infection may be overlooked. The manifestations can be broadly categorized by the primary systems affected.
1.3.1 Neurological and Psychiatric Sequelae
The central and peripheral nervous systems are frequent targets in chronic bartonellosis. The constellation of neurological and psychiatric symptoms is often the most debilitating aspect of the disease.
Neurological Symptoms: Patients commonly report persistent and severe headaches that can be migraine-like, cognitive dysfunction often described as "brain fog" (encompassing memory loss, poor concentration, and disorientation), dizziness, vertigo, tremors, ataxia (poor coordination), and visual disturbances such as blurred vision or floaters.3 In more severe cases, seizures and other focal neurological deficits have been documented.26
Psychiatric Symptoms: The psychiatric manifestations of bartonellosis can be profound and are often resistant to standard psychopharmacological treatments, a key clinical clue that an underlying organic process may be at play.28 Common symptoms include severe anxiety, panic attacks, treatment-resistant depression, emotional lability, irritability, and episodes of agitation or uncharacteristic rage.13 In a subset of patients, more severe psychiatric syndromes, including psychosis and hallucinations, have been reported.13
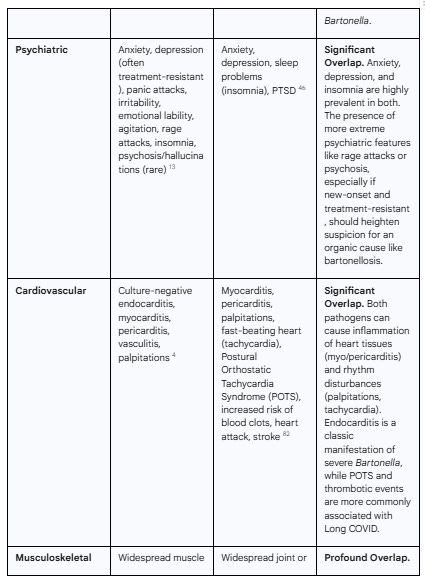
1.3.2 Cardiovascular Involvement
The affinity of Bartonella for endothelial cells makes the cardiovascular system a primary site of pathology.34
Endocarditis: A hallmark of severe Bartonella infection is culture-negative endocarditis, an infection of the heart valves that is notoriously difficult to diagnose with standard blood cultures.1 It can lead to severe valvular damage requiring surgical replacement.
Other Cardiac and Vascular Manifestations: Beyond the heart valves, Bartonella can cause myocarditis (inflammation of the heart muscle), pericarditis (inflammation of the sac surrounding the heart), and systemic vasculitis (inflammation of blood vessels).13 In immunocompromised individuals, it can cause vasoproliferative lesions known as bacillary angiomatosis, which are tumor-like masses of new blood vessels.1
1.3.3 Musculoskeletal and Constitutional Symptoms
The systemic and persistent nature of the infection frequently leads to a range of constitutional and musculoskeletal complaints that significantly impact quality of life.
Constitutional Symptoms: Debilitating fatigue and malaise (a general feeling of being unwell) are cornerstone symptoms of chronic bartonellosis.1 Other common findings include relapsing low-grade fevers, night sweats, and persistently swollen lymph nodes (lymphadenopathy).1
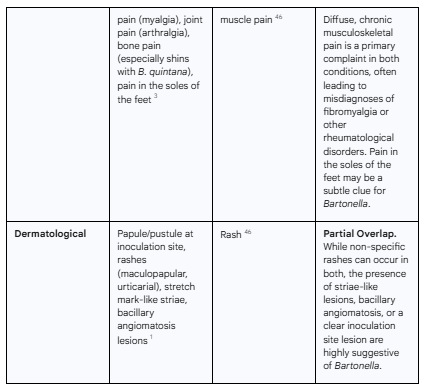
Musculoskeletal Symptoms: Widespread and often migratory muscle pain (myalgia) and joint pain (arthralgia) are frequently reported, sometimes leading to a misdiagnosis of rheumatological disorders like fibromyalgia or rheumatoid arthritis.24 A characteristic, though not universal, symptom is sharp pain in the soles of the feet, particularly upon waking in the morning.3 Bone pain, especially in the shins, is a classic feature of trench fever caused by
B. quintana.3Dermatological Symptoms: Skin manifestations can include the initial papule or pustule at the site of inoculation, rashes, and distinctive stretch mark-like lesions (striae), which are often reddish or purplish in color.1
This broad and often non-specific clinical picture establishes chronic bartonellosis as a great mimicker of other diseases, setting the stage for the diagnostic challenges that arise when its symptoms overlap with those of another complex, multisystemic condition.
Section 2: The Enigma of Long COVID: A Multisystem Post-Infectious Syndrome
Long COVID, or PASC, has emerged as a formidable challenge in the wake of the SARS-CoV-2 pandemic. It represents a complex, multisystemic disorder that can persist for months or years, profoundly affecting an individual's health and quality of life. Understanding the clinical definition, proposed biological mechanisms, and diverse symptom profile of Long COVID is essential to contextualize how a latent bacterial infection like bartonellosis might intersect with and contribute to this condition.
2.1 Defining the Condition: Prevalence and Heterogeneity
Long COVID is broadly defined as a chronic condition that occurs after SARS-CoV-2 infection, characterized by signs and symptoms that are present for at least three months.41 The condition is not a single, uniform illness but rather a heterogeneous syndrome with a wide spectrum of presentations. Symptoms can be continuous, they can relapse and remit over time, or they can be progressive, worsening as months pass.42
The global burden of Long COVID is substantial. While estimates vary, studies suggest that 10-20% of individuals infected with SARS-CoV-2 may go on to develop persistent symptoms, translating to millions of affected individuals worldwide.41 A critical and often misunderstood aspect of Long COVID is that its development is not contingent on the severity of the initial COVID-19 illness. It can, and frequently does, occur following mild or even asymptomatic infections.41 While anyone can develop Long COVID, research has identified several risk factors, including being female, having pre-existing underlying health conditions (such as Type 2 diabetes), experiencing a high number of symptoms during the acute phase, and being unvaccinated against COVID-19.42 The condition's heterogeneity is underscored by the identification of more than 200 different symptoms affecting nearly every organ system.41
2.2 Core Pathophysiological Hypotheses
The pathophysiology of Long COVID is not a single, settled entity but rather a complex interplay of multiple potential mechanisms. The scientific consensus is coalescing around several core hypotheses, with immune dysregulation and endothelial damage emerging as central, unifying pillars that can help explain the condition's pervasive, multisystemic nature. These biological disruptions are precisely what could create a permissive environment for the reactivation of a latent pathogen.
The leading hypotheses include:
Viral Persistence and Antigenic Reservoirs: A growing body of evidence suggests that the SARS-CoV-2 virus or its components, such as the S1 spike protein, may not be fully cleared from the body after the acute phase. These viral remnants can persist in various tissues, including the gut, nervous system, and endothelial cells, acting as a chronic antigenic stimulus that drives ongoing inflammation and immune activation.42
Immune Dysregulation and Autoimmunity: SARS-CoV-2 infection can trigger profound and lasting disruptions to the immune system. The acute phase can be marked by a "cytokine storm"—an overproduction of inflammatory signaling molecules—as well as lymphopenia (a depletion of T-lymphocytes) and T-cell exhaustion.61 This dysregulation can persist into the chronic phase, leading to a state of sustained inflammation.58 Furthermore, the infection can induce the production of autoantibodies, which are antibodies that mistakenly target and attack the body's own tissues, leading to autoimmune phenomena that contribute to Long COVID symptoms.42
Endotheliopathy and Microvascular Thrombosis: Widespread inflammation and damage to the endothelial cells lining the blood vessels (a condition known as endotheliitis or endotheliopathy) is a central feature of both acute and long-term COVID-19.67 This damage impairs vascular function, promotes the formation of microscopic blood clots (microthrombosis), and reduces the efficient delivery of oxygen to tissues throughout the body.51 This microvascular pathology is a strong candidate for explaining many of the systemic symptoms of Long COVID, from cognitive dysfunction to fatigue.
Reactivation of Latent Pathogens: The immune dysregulation caused by SARS-CoV-2 can compromise the immune system's ability to keep other, previously acquired latent pathogens in a dormant state. The reactivation of herpesviruses, most notably the Epstein-Barr virus (EBV), has been well-documented in Long COVID patients and is considered a significant risk factor for developing the condition.51 This established phenomenon provides a direct biological precedent for the hypothesis that SARS-CoV-2 could similarly reactivate a latent
Bartonella infection.
It is crucial to recognize that Long COVID shares many clinical and pathophysiological features with other post-infectious syndromes, such as ME/CFS and Post-Treatment Lyme Disease Syndrome.43 This placement within a known class of illnesses suggests that lessons learned from decades of research into other complex, post-infectious conditions—including the potential role of persistent or reactivated co-infections—may be directly applicable to understanding and treating a subset of Long COVID cases.78
2.3 The Broad Symptom Profile of Long COVID
The symptom profile of Long COVID is remarkably broad and mirrors the multisystemic nature of chronic bartonellosis, a fact that lies at the heart of the diagnostic challenge this report addresses.
2.3.1 Neurological and Psychiatric Manifestations
Neurological and psychiatric symptoms are among the most prevalent and life-altering aspects of Long COVID.
Neurological Symptoms: The most widely recognized neurological symptom is "brain fog," a colloquial term for a constellation of cognitive deficits including impaired memory, difficulty concentrating, and slowed information processing.41 Other common neurological complaints include persistent headaches, debilitating sleep disorders (insomnia), dizziness (particularly upon standing), and peripheral neuropathies manifesting as "pins-and-needles" sensations.46 A new or altered sense of smell or taste is also a characteristic feature.51
Psychiatric Symptoms: High rates of anxiety, depression, and post-traumatic stress disorder (PTSD) are documented in the Long COVID population, stemming from both the biological effects of the illness on the brain and the psychological burden of living with a chronic, disabling condition.46
2.3.2 Cardiovascular Complications
The cardiovascular system is significantly impacted in Long COVID, reflecting the underlying endotheliopathy and inflammation.
Common Symptoms: Patients frequently report heart palpitations, a rapid or pounding heartbeat (tachycardia), and chest pain.51
Specific Conditions: Long COVID is associated with an increased risk of developing specific cardiovascular conditions, including myocarditis, pericarditis, arrhythmias, heart failure, and thromboembolic events such as heart attack and stroke.82 A notable complication is Postural Orthostatic Tachycardia Syndrome (POTS), a form of dysautonomia characterized by an abnormal increase in heart rate upon standing.56 Studies have also shown that COVID-19 can lead to "early vascular aging," characterized by increased arterial stiffness.90
2.3.3 Musculoskeletal and Constitutional Symptoms
Constitutional and musculoskeletal symptoms are often the most prominent and disabling features of Long COVID.
Constitutional Symptoms: The hallmark symptom is a profound and persistent fatigue that is often described as qualitatively different from normal tiredness and is not relieved by rest.41 A key feature shared with ME/CFS is post-exertional malaise (PEM), a severe worsening of symptoms following even minor physical or mental exertion.45
Musculoskeletal Symptoms: Widespread and often chronic muscle pain (myalgia) and joint pain (arthralgia) are very common, contributing significantly to functional disability.46
The striking parallels between this symptom profile and that of chronic bartonellosis form the clinical foundation for the hypothesis that these two conditions may be linked in a subset of patients.
Section 3: Pathophysiological Convergence: Shared Mechanisms of Chronic Illness
The clinical similarities between chronic bartonellosis and Long COVID are not coincidental; they are rooted in a deep and compelling convergence of their underlying pathophysiological mechanisms. Both SARS-CoV-2 and Bartonella species are pathogens that profoundly disrupt the host's immune and vascular systems. By analyzing these shared pathways, a strong theoretical framework emerges that not only explains the symptom overlap but also provides a biologically plausible basis for how an acute viral infection could reactivate a latent bacterial one.
3.1 Immune Dysregulation: A Common Ground for Pathogen Reactivation
The most critical point of convergence is the impact both pathogens have on the host immune system. This shared mechanism provides the foundation for the central reactivation hypothesis.
The immune dysregulation induced by SARS-CoV-2 is well-documented. The acute phase of COVID-19 can be characterized by a profound disruption of immune homeostasis, including lymphopenia (a significant reduction in T-cell counts, particularly CD4+ and CD8+ cells), functional exhaustion of surviving T-cells, and a hyperinflammatory state often termed a "cytokine storm".61 This state of immune chaos is not always transient. In individuals who develop Long COVID, evidence suggests this dysregulation can persist for months or even years, leading to chronic inflammation and a compromised ability to effectively manage other pathogens.58
This creates a state of functional, if not absolute, immunodeficiency. While not as severe as the immunosuppression seen in conditions like advanced HIV, the disruption is significant enough to lower the threshold of immune surveillance that normally keeps latent pathogens in check. This is precisely the scenario required for an opportunistic pathogen to emerge. The concept of "opportunistic infection" may need to be broadened in the post-COVID era; it is no longer restricted to cases of profound immune collapse. The more subtle, yet chronic, immune dysregulation induced by SARS-CoV-2 appears sufficient to allow stealth pathogens like Bartonella to reactivate.
This hypothesis is strongly supported by the well-established phenomenon of herpesvirus reactivation in Long COVID. Multiple studies have documented the reactivation of latent viruses like Epstein-Barr virus (EBV) and varicella-zoster virus in Long COVID patients, and this reactivation is considered a key risk factor for the development of chronic symptoms.51 The biological logic is identical: a primary viral infection (SARS-CoV-2) disrupts the immune system, allowing a secondary, latent pathogen (EBV or, as proposed here, Bartonella) to switch from a dormant to a replicative state, thereby contributing to the patient's chronic symptom burden. Bartonella, as a master of immune evasion that thrives by hiding from a functional immune system, is an ideal candidate for this type of reactivation event.20
3.2 The Endothelium as a Shared Battlefield: A Comparative Analysis of Vascular Injury
The second major area of pathophysiological convergence is the vascular endothelium—the single layer of cells lining all blood vessels. This delicate tissue is a primary target for both SARS-CoV-2 and Bartonella species, making it a shared battlefield where the pathologies of both infections can intersect and potentially synergize.
Endothelial dysfunction is now recognized as a central factor in the progression of both acute COVID-19 and Long COVID.67 SARS-CoV-2 can damage the endothelium both directly, by infecting cells that express its ACE2 receptor, and indirectly, through the massive inflammatory response it triggers.67 This damage leads to a state of endotheliitis, characterized by increased vascular permeability, a pro-thrombotic state (promoting blood clots), impaired vasodilation due to reduced nitric oxide bioavailability, and widespread microcirculatory dysfunction.59 This systemic vascular pathology is thought to underlie many of Long COVID's most pervasive symptoms, including brain fog, fatigue, and cardiovascular complications.
Similarly, Bartonella species are classic endothelial pathogens. Their lifecycle is intimately tied to the vasculature. They infect and persist within endothelial cells, using them as a protected niche from which to seed the bloodstream.14 In doing so, they can induce a range of pathological changes, from inhibiting endothelial cell apoptosis to, in immunocompromised hosts, stimulating the rampant proliferation of blood vessels (bacillary angiomatosis).23
This shared tropism for the endothelium suggests the potential for a synergistic and bidirectional interaction. The relationship may be more complex than a simple one-way reactivation. A pre-existing, latent Bartonella infection, with its associated low-grade inflammation and colonization of endothelial cells, could "prime" the host for a more severe reaction to a subsequent SARS-CoV-2 infection. The virus, upon entering the body, would encounter an already compromised endothelial landscape. The resulting acute inflammatory assault and endothelial damage could be massively amplified, leading to a more severe case of COVID-19 and a higher likelihood of progressing to Long COVID. This, in turn, creates a vicious cycle: the pre-existing Bartonella worsens the acute COVID response, and the severe immune dysregulation resulting from that response unleashes the now-uncontrolled latent Bartonella. This model helps to explain the heterogeneity of Long COVID outcomes and moves beyond a simple reactivation hypothesis to one of synergistic pathogenesis.
3.3 Inflammatory Pathways and Cytokine Signatures in Bartonellosis and Long COVID
Both chronic bartonellosis and Long COVID are fundamentally inflammatory conditions. While the specific cytokine signatures are still being fully elucidated, the general pathways show considerable overlap. Long COVID is associated with persistently elevated levels of pro-inflammatory cytokines such as Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α), and various chemokines.66 These signaling molecules perpetuate a state of chronic inflammation that drives tissue damage and symptoms.
Bartonella infection also induces a complex inflammatory response. While the bacteria have evolved ways to modulate this response to their advantage, their presence is a constant stimulus for the immune system.20 Therefore, it is plausible that the persistent inflammation observed in a subset of Long COVID patients is not solely due to a SARS-CoV-2 reservoir or autoimmunity, but is being actively fueled by a co-existing, reactivated Bartonella infection. The two pathogens could be contributing to a shared pool of inflammatory mediators, creating a sustained inflammatory state that is more severe and persistent than either pathogen would cause alone. Unraveling these specific inflammatory pathways will be a critical area for future research.
Section 4: Clinical Evidence and Symptomatic Overlap
While the mechanistic plausibility is strong, the hypothesis linking bartonellosis and Long COVID is ultimately supported by direct clinical evidence. This evidence comes from a growing number of case reports where Bartonella has been identified in patients with a Long COVID diagnosis, as well as from the profound and undeniable overlap in the symptom profiles of the two conditions. This overlap creates a significant risk of diagnostic confusion and overshadowing, where the new and prominent diagnosis of Long COVID may prevent clinicians from considering an underlying, treatable bacterial cause.
4.1 Analysis of Case Reports: Unmasking and Reactivating Bartonella in the Post-COVID Era
The published case reports, though limited in number, are pivotal as they provide direct, in-human evidence of the co-occurrence and interaction of these two diseases. A striking pattern that emerges from these cases is that Long COVID can function as a "diagnostic unmasking event." Patients with what may have been vague, chronic symptoms from a previously undiagnosed Bartonella infection are driven into the medical system by an acute COVID-19 infection. Their subsequent persistent symptoms are initially, and reasonably, labeled as Long COVID. It is only through persistent investigation, often involving advanced diagnostic technologies, that the underlying bacterial infection is revealed.
Case 1: "Unmasking Bartonella henselae infection in the shadows of long COVID".98 This seminal case involved a 26-year-old woman who, following a mild COVID-19 infection, developed classic Long COVID symptoms, including a persistent cough, exertional dyspnea, night sweats, significant weight loss, and tender axillary lymphadenopathy. A comprehensive workup for her post-COVID symptoms, including a PET scan, was suggestive of lymphoma but ultimately inconclusive. The diagnostic breakthrough came from a lymph node biopsy subjected to clinical metagenomics—an unbiased, deep-sequencing approach. This analysis revealed the presence of Bartonella henselae DNA and RNA, confirming an active infection. Crucially, upon initiation of targeted antibiotic therapy with clarithromycin, the patient's symptoms improved. This case is a paradigm example of Bartonella infection perfectly mimicking Long COVID and being missed by conventional diagnostic pathways, only to be "unmasked" by advanced molecular testing.
Case 2: "Post-COVID reactivation of latent Bartonella henselae infection".102 This report details the case of a 54-year-old male who had a painful mass on his arm that was gradually resolving. Immediately following an infection with COVID-19, the mass rapidly progressed into a suppurating abscess, and he simultaneously developed new, severe pulmonary symptoms, including pleural effusion. Routine cultures and pathology were unrevealing. The diagnosis of B. henselae was only confirmed when tissue from the abscess was analyzed using Next-Generation Sequencing (NGS). This case provides powerful, direct evidence for the reactivation hypothesis, demonstrating how an acute COVID-19 infection can act as a potent trigger, transforming a contained, latent bacterial process into an aggressive, systemic illness.
Case 3: "A puzzling multisystem disorder complicated by nosocomial COVID-19 infection".106 This case describes a 60-year-old man who presented with a perplexing multisystem illness including chest pain, fever, rash, and pericardial effusion. The cause was not immediately apparent until serological testing confirmed a Bartonella infection. His hospital course was further complicated when he contracted a nosocomial (hospital-acquired) COVID-19 infection, requiring intensive care. This case highlights the clinical complexity of co-infection and illustrates how one severe illness can obscure or dramatically worsen the course of another.
Case 4: Clinical Vignette of Long COVID and Suspected Co-infections.107 A case study of a 26-year-old medical student with a classic Long COVID presentation—including headaches, POTS-like symptoms, brain fog, and fatigue—who was also diagnosed with Lyme disease. The author, a physician specializing in tick-borne diseases, noted that several of her specific symptoms, such as visual floaters, night terrors, and phantosmia (olfactory hallucinations), are highly characteristic of a Bartonella co-infection. While not diagnostically confirmed in the report, this case reflects the growing clinical suspicion among experts in the field that a subset of Long COVID cases involves underlying vector-borne infections.
4.2 Observational Data: Seroprevalence and Co-Infection Insights
Beyond individual case reports, larger-scale observational data provide additional context. A study conducted in Denver, Colorado, during a B. quintana outbreak among persons experiencing homelessness analyzed residual serum samples that had been collected for SARS-CoV-2 antibody testing.108 The study found that approximately 15% of this population was seroreactive to Bartonella. The analysis did not find a statistically significant association between being seropositive for SARS-CoV-2 and being seropositive for Bartonella.
A nuanced interpretation of these findings is essential. The lack of a statistical association does not disprove the reactivation hypothesis. Seroreactivity indicates only past exposure and the presence of antibodies; it does not differentiate between a past cleared infection, a latent controlled infection, or an active infection. The key trigger for reactivation, as proposed in this report, is the acute inflammatory and immune-dysregulating event of the COVID-19 illness itself, not simply a history of prior exposure to both pathogens. The study's cross-sectional design and reliance on serology limit its ability to assess causality or the dynamics of reactivation. However, it does confirm the co-circulation of both pathogens in a vulnerable population, establishing the epidemiological opportunity for co-infection and interaction to occur.
4.3 A Comparative Symptomatology: Distinguishing Signal from Noise
The most significant challenge for clinicians is the near-total overlap in the most common and debilitating symptoms of chronic bartonellosis and Long COVID. This makes clinical differentiation based on symptom presentation alone highly unreliable and creates a substantial risk of diagnostic overshadowing. When a patient presents with fatigue, brain fog, and pain following a confirmed or suspected SARS-CoV-2 infection, the default diagnosis will be Long COVID. This may prematurely halt the diagnostic process, preventing the consideration of a treatable bacterial cause.
The following table provides a systematic, side-by-side comparison of the symptom profiles of both conditions, compiled from the extensive clinical descriptions in the source material. It serves to visually demonstrate the profound degree of overlap.
Table 1: Comparative Analysis of Multisystemic Symptoms in Chronic Bartonellosis and Long COVID
As the table demonstrates, the overlap is not partial; it is pervasive. The core, most disabling symptoms that define the patient experience in both conditions—fatigue, cognitive impairment, and widespread pain—are virtually indistinguishable. This clinical reality underscores the inadequacy of a purely symptom-based diagnosis and powerfully argues for the need to incorporate objective, pathogen-directed testing into the workup of complex post-COVID illness.
Section 5: Diagnostic and Therapeutic Implications for Clinical Practice
The convergence of pathophysiology and clinical presentation between chronic bartonellosis and Long COVID carries profound implications for clinical practice. The current diagnostic paradigm for Long COVID, which is largely a diagnosis of exclusion, may be inadvertently excluding too little. The failure to consider and adequately test for a treatable bacterial pathogen like Bartonella represents a significant potential pitfall in patient care. This section translates the analytical findings of this report into actionable guidance for clinicians, addressing the diagnostic challenges and proposing a path forward for both testing and treatment.
5.1 The Diagnostic Challenge: Why Bartonella May Be Missed in Long COVID Workups
Several factors conspire to make Bartonella a "hidden" infection, easily missed in the standard workup for a patient with post-COVID symptoms.
Insufficiency of Standard Culture: Bartonella species are notoriously difficult to isolate using conventional blood culture methods. They are fastidious, slow-growing, and primarily intracellular bacteria. Standard blood cultures, typically incubated for five days, will almost always be negative. Successful culture requires specialized media and extended incubation periods of 21 days or more, a protocol that must be specifically requested and is not routinely performed.9 This creates a high rate of false-negative results, leading clinicians to incorrectly rule out an active bacterial infection.
Limitations of Serology: Serological tests, which detect host antibodies to the bacteria, are more commonly used but have significant limitations that can complicate interpretation.
Cross-Reactivity: Antibodies may cross-react between different Bartonella species or even with other unrelated bacteria, reducing specificity.9
Persistence: IgG antibody titers can remain elevated for years following a past, resolved infection, making it impossible to distinguish a prior exposure from a current, active infection based on a single test.9 While seroconversion (a change from negative to positive or a significant rise in titer) between acute and convalescent samples is strong evidence of recent infection, this is often not practical in a chronic illness setting.
False Negatives: Some patients with active, PCR-confirmed bacteremia may not mount a detectable antibody response and will be seronegative, leading to false-negative results.26
The Promise of Advanced Molecular Diagnostics: The case reports that successfully identified Bartonella in Long COVID patients highlight a crucial point: advanced molecular diagnostics represent a necessary paradigm shift. Techniques like Polymerase Chain Reaction (PCR), droplet digital PCR (ddPCR), and especially unbiased Next-Generation Sequencing (NGS) or metagenomics offer vastly superior sensitivity and specificity for direct pathogen detection.9 These methods are particularly powerful when applied to tissue samples (e.g., lymph node biopsy, resected heart valve) or after using enrichment culture techniques (like Bartonella Alpha Proteobacteria Growth Medium, or BAPGM) to increase the bacterial load in blood or fluid samples prior to PCR.12 The successful use of these tools in the case reports is not a minor technical detail; it demonstrates that for these complex, "mystery" illnesses, an agnostic, molecular approach that can identify any pathogen present may be required to find the correct diagnosis.
Diagnostic Overshadowing by Long COVID: The emergence of Long COVID as a recognized, widespread syndrome has created a powerful cognitive bias in clinical practice. Because Long COVID itself has no definitive biomarker and is diagnosed based on a history of COVID-19 followed by persistent symptoms, there is a tendency to attribute any and all post-COVID symptoms to this new entity.43 This "diagnostic overshadowing" can prematurely halt the investigation for other underlying or contributing causes, leaving a potentially treatable infection like bartonellosis undiscovered.
5.2 A Proposed Diagnostic Algorithm for Atypical or Refractory Long COVID
Given these challenges, a more structured and suspicious approach is warranted for a subset of patients carrying a Long COVID diagnosis. Clinicians should consider pursuing a workup for chronic Bartonella infection in patients who meet certain criteria.
Criteria for Initiating Bartonella Investigation in a Long COVID Patient:
Refractory Symptoms: The patient's symptoms are not improving or are worsening over time despite standard supportive care for Long COVID.
Atypical or Severe Feature Dominance: The clinical picture is dominated by features that, while seen in Long COVID, are also classic for severe bartonellosis. These include:
Severe, treatment-resistant neurological or psychiatric symptoms (e.g., severe migraines, cognitive decline, new-onset panic/rage).
Clinical or imaging evidence of endocarditis, myocarditis, or pericarditis.
Persistent, tender lymphadenopathy.
Specific dermatological signs (e.g., striae-like lesions, suspected bacillary angiomatosis).
Relevant Exposure History: While not required (as exposure may be unknown or forgotten), a history of cat scratches, flea bites, or other potential vector exposures should increase the index of suspicion.
Recommended Diagnostic Testing Cascade:
Step 1: High-Quality Serology: Begin with an indirect immunofluorescence assay (IFA) for IgG antibodies against multiple Bartonella species, primarily B. henselae and B. quintana. A positive result, while not definitive of active infection, confirms exposure and justifies further testing.
Step 2: Direct Detection via Enhanced Molecular Methods: If serology is positive or if clinical suspicion remains high despite negative serology, proceed to direct detection methods. The preferred approach is PCR testing on a sample that has been enriched using a specialized culture medium like BAPGM. This significantly increases the yield compared to standard blood PCR.71
Step 3: Tissue Biopsy and Molecular Analysis: In patients with localized findings, a tissue biopsy is the highest-yield diagnostic procedure. An enlarged lymph node, a suspicious skin lesion, or (in cases of cardiac surgery) resected heart valve tissue should be sent for both histopathology (with Warthin-Starry silver stain) and, most importantly, broad-range 16S rRNA PCR and/or NGS/metagenomic analysis.9
5.3 Emerging Therapeutic Considerations and the Need for Clinical Trials
The most compelling reason to pursue this challenging diagnosis is that, unlike Long COVID, bartonellosis is a bacterial infection with the potential for effective treatment. The case reports demonstrate that when an underlying Bartonella infection is identified and treated with appropriate antibiotics—such as macrolides (clarithromycin, azithromycin), tetracyclines (doxycycline), or rifampin—significant clinical improvement can occur.98
However, a major evidence gap exists. There is currently no universally accepted, standard treatment protocol for chronic or disseminated bartonellosis, and optimal drug combinations and durations of therapy are subjects of ongoing debate.115 This situation mirrors the long-standing controversies in the treatment of persistent Lyme disease, where patient and physician experiences often diverge from restrictive official guidelines.50
This highlights a critical need for rigorous clinical trials. The ideal study would be a prospective, randomized controlled trial that enrolls a large cohort of patients meeting the criteria for Long COVID. These patients would undergo comprehensive testing for Bartonella using the advanced molecular methods described above. Those found to have an active infection would then be randomized to receive either a prolonged course of combination antibiotic therapy or a placebo, with standardized symptom and quality-of-life metrics tracked over time. Such a trial would provide the definitive evidence needed to confirm or refute the hypothesis that treating underlying bartonellosis can resolve symptoms in a subset of the Long COVID population.
Section 6: Future Directions and Recommendations
The emerging evidence linking Bartonella infection and Long COVID, while still in its infancy, points toward a potentially significant area of clinical and scientific inquiry. Addressing this intersection requires a coordinated effort from researchers, clinicians, and public health agencies. A failure to investigate this potential link represents a significant missed opportunity to provide effective, targeted treatment for a subset of patients currently suffering under the broad and often intractable diagnosis of Long COVID.
6.1 Priority Areas for Biomedical Research
To move from case reports and mechanistic plausibility to robust, evidence-based conclusions, a focused research agenda is imperative. The investigation into the Bartonella-Long COVID link could serve as a valuable model for understanding the broader phenomenon of infection-associated chronic illness, leveraging the unprecedented funding and attention directed toward Long COVID to solve mysteries that have long plagued related conditions like ME/CFS and post-treatment Lyme disease syndrome.50
Key research priorities should include:
Prospective Cohort Studies: Large-scale, longitudinal studies of Long COVID patients are urgently needed. These studies must incorporate comprehensive, state-of-the-art testing for Bartonella and other potential latent pathogens at baseline and at regular follow-up intervals. Utilizing platforms like the NIH's RECOVER Initiative, which has already enrolled a massive and diverse cohort, would be an efficient way to integrate this research.54 Such studies would allow for the determination of prevalence, risk factors, and clinical outcomes of co-infection.
Development of Reliable Biomarkers: A major barrier to both clinical care and research is the difficulty in diagnosing active bartonellosis. A high-priority goal must be the development and validation of more reliable, non-invasive diagnostic tests—whether molecular, antigenic, or immunological—that can accurately distinguish active, persistent infection from past exposure.
Mechanistic and Preclinical Studies: Basic science research is needed to elucidate the precise molecular mechanisms at play. This includes using animal models and advanced cell culture systems to directly investigate how SARS-CoV-2 infection alters the host immune response to a latent Bartonella infection and how the two pathogens might synergistically damage the vascular endothelium.
Randomized Controlled Clinical Trials: As outlined in the previous section, well-designed clinical trials are the ultimate arbiter of therapeutic efficacy. Trials testing targeted antibiotic regimens in Long COVID patients with confirmed active Bartonella infection are essential to determine if this approach leads to tangible clinical improvement.
Leveraging Existing Expertise: Research efforts should build upon the work of institutions and investigators already at the forefront of studying complex chronic illnesses. Centers like Mount Sinai's Cohen Center for Recovery from Complex Chronic Illnesses, organizations like the PolyBio Research Foundation, and academic researchers at institutions like Columbia University and the University of Arizona are already exploring the intersections of Long COVID, ME/CFS, and vector-borne diseases, providing a valuable foundation of expertise.81
6.2 Recommendations for Clinicians and Public Health Agencies
The clinical and ethical imperative to investigate this potential link is high. While awaiting definitive data from large-scale studies, clinicians and public health bodies can take immediate steps based on the current evidence.
Recommendations for Clinicians:
Maintain a High Index of Suspicion: In patients with a Long COVID diagnosis who are not improving or who present with atypical or severe neurological, psychiatric, or cardiovascular symptoms, Bartonella infection should be actively considered in the differential diagnosis.
Educate on Diagnostic Limitations: Clinicians should become familiar with the significant limitations of standard blood culture and serology for diagnosing chronic bartonellosis and understand that a negative result from these tests does not definitively rule out active infection.
Utilize Advanced Diagnostics: When suspicion is high, clinicians should seek out and utilize advanced molecular testing, such as PCR on enriched blood cultures or NGS/metagenomics on tissue biopsies where appropriate, as these methods offer the highest diagnostic yield.
Acknowledge Symptom Overlap: Recognize that the symptom profiles of Long COVID and chronic bartonellosis are nearly identical for the most common complaints. This awareness should prevent the premature closure of a diagnostic workup based on the Long COVID label alone.
Recommendations for Public Health Agencies (e.g., CDC, WHO):
Update Clinical Guidance: Official clinical guidance documents for the evaluation and management of Long COVID should be updated to include the consideration of reactivated latent infections, including Bartonella, as a potential contributing factor in the differential diagnosis of patients with persistent symptoms.42
Promote Clinician Education: Agencies should develop and disseminate educational materials for healthcare providers that highlight the diverse clinical presentations of bartonellosis, the challenges in its diagnosis, and its potential connection to post-viral syndromes.
Support Research and Surveillance: Public health bodies should support and fund the priority research areas outlined above, particularly large-scale surveillance studies to determine the prevalence of Bartonella co-infection in the Long COVID population.
Conclusion
The evidence, though emerging, paints a compelling picture of a potential and clinically significant relationship between Bartonella infection and Long COVID. The convergence of multiple lines of reasoning—the biological plausibility of reactivation driven by SARS-CoV-2-induced immune dysregulation, the shared pathophysiological targeting of the vascular endothelium, the profound overlap in clinical symptomatology, and the direct evidence from meticulously investigated case reports—collectively argues that this association warrants urgent and systematic investigation.
For a subset of the millions struggling with Long COVID, the true culprit of their suffering may not be a lingering ghost of the SARS-CoV-2 virus, but rather an older, more insidious pathogen that was awakened by the pandemic. The failure to recognize this possibility risks condemning these patients to a cycle of supportive care for a condition deemed intractable, when a targeted, curative treatment may be available. The clinical and scientific communities have a responsibility to look deeper, to question assumptions, and to employ the full power of modern molecular diagnostics to unravel the complex biology of post-infectious illness. The challenge now is to move beyond isolated case reports and toward a comprehensive research program that can definitively clarify the role of Bartonella in the long shadow of COVID-19. Uncovering this hidden adversary could provide not only answers and effective therapies for a subset of Long COVID patients but also invaluable insights into the broader universe of infection-associated chronic disease.
Acknowledgement
I acknowledge the assistance of Gemini AI in the preparation of the subject research plan, the execution of the research, and the preparation of this report.
Works cited
Bartonellosis - Symptoms, Causes, Treatment - National Organization for Rare Disorders, accessed August 18, 2025, https://rarediseases.org/rare-diseases/bartonellosis/
Bartonellosis - Wikipedia, accessed August 18, 2025, https://en.wikipedia.org/wiki/Bartonellosis
Bartonellosis - StatPearls - NCBI Bookshelf, accessed August 18, 2025, https://www.ncbi.nlm.nih.gov/books/NBK430874/
About Bartonella henselae - CDC, accessed August 18, 2025, https://www.cdc.gov/bartonella/about/about-bartonella-henselae.html
About Bartonella - CDC, accessed August 18, 2025, https://www.cdc.gov/bartonella/about/index.html
Bartonellosis: Causes, Symptoms, Treatment & Prevention - Cleveland Clinic, accessed August 18, 2025, https://my.clevelandclinic.org/health/diseases/bartonellosis
Can You Get Rabies From a Cat Scratch? - Verywell Health, accessed August 18, 2025, https://www.verywellhealth.com/what-did-the-cat-can-drag-in-1958916
GUIDELINE for Feline bartonellosis - ABCD cats & vets, accessed August 18, 2025, https://www.abcdcatsvets.org/guideline-for-feline-bartonellosis/
Clinical Guidance for Bartonella quintana - CDC, accessed August 18, 2025, https://www.cdc.gov/bartonella/hcp/bartonella-quintana/index.html
About Bartonella quintana - CDC, accessed August 18, 2025, https://www.cdc.gov/bartonella/about/about-bartonella-quintana.html
Bartonella quintana Infection in Canada: A Retrospective Laboratory Study and Systematic Review of the Literature - MDPI, accessed August 18, 2025, https://www.mdpi.com/2076-0817/13/12/1071
What is Bartonellosis? - Galaxy Diagnostics, accessed August 18, 2025, https://www.galaxydx.com/what-is-bartonellosis/
Human Bartonellosis: An Underappreciated Public Health Problem? - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6630881/
(PDF) Pestilence, Persistence and Pathogenicity: Infection Strategies of Bartonella, accessed August 18, 2025, https://www.researchgate.net/publication/26721556_Pestilence_persistence_and_pathogenicity_Infection_strategies_of_Bartonella
Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation, accessed August 18, 2025, https://academic.oup.com/femsre/article-pdf/36/3/563/18129086/36-3-563.pdf
Molecular Mechanisms of Bartonella and Mammalian Erythrocyte Interactions: A Review - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6299047/
Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation | FEMS Microbiology Reviews | Oxford Academic, accessed August 18, 2025, https://academic.oup.com/femsre/article/36/3/563/634924
Advancements in understanding the molecular and immune mechanisms of Bartonella pathogenicity - ResearchGate, accessed August 18, 2025, https://www.researchgate.net/publication/371872480_Advancements_in_understanding_the_molecular_and_immune_mechanisms_of_Bartonella_pathogenicity
Advancements in understanding the molecular and immune mechanisms of Bartonella pathogenicity - Frontiers, accessed August 18, 2025, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2023.1196700/full
Immune sera interfere with Bartonella adhesion to erythrocytes while... | Download Scientific Diagram - ResearchGate, accessed August 18, 2025, https://www.researchgate.net/figure/mmune-sera-interfere-with-Bartonella-adhesion-to-erythrocytes-while-complement-and_fig2_361384026
Sneaky tactics: Ingenious immune evasion mechanisms of Bartonella - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10936683/
Full article: Sneaky tactics: Ingenious immune evasion mechanisms of Bartonella, accessed August 18, 2025, https://www.tandfonline.com/doi/full/10.1080/21505594.2024.2322961
Detrimental effects of Bartonella henselae are counteracted by l-arginine and nitric oxide in human endothelial progenitor cells | PNAS, accessed August 18, 2025, https://www.pnas.org/doi/10.1073/pnas.0803602105
Long-Term Bartonella Symptoms, Key Signs - Lyme Mexico Clinic, accessed August 18, 2025, https://lymemexico.com/experiencing-long-term-bartonella-symptoms-sign/
Bartonella - Project Lyme, accessed August 18, 2025, https://projectlyme.org/resource/what-is-bartonella/
Bartonella sp. Bacteremia in Patients with Neurological and Neurocognitive Dysfunction - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2546763/
Supplement - Bartonella Infections in Humans: Clinical Signs, accessed August 18, 2025, https://www.cfsph.iastate.edu/DiseaseInfo/pdf/bartonella-infections-in-humans-supplement.pdf
Bartonella Psychiatric & Neurological Symptoms Fort Meyers FL - James Schaller MD, MAR, accessed August 18, 2025, https://www.personalconsult.com/services/bartonella/bartonella-psychiatric-symptoms-neurological-symptoms/
Do Bartonella Infections Cause Agitation, Panic Disorder, and Treatment-Resistant Depression? - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2100128/
Bartonella Symptoms & How To Recognize Bartonellosis - Medicine With Heart Institute, accessed August 18, 2025, https://mindbodyfunctionalmedicine.com/blog/bartonella-symptoms-how-to-recognize-bartonellosis/
pmc.ncbi.nlm.nih.gov, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2100128/#:~:text=In%20this%20article%2C%20we%20discuss,may%20be%20attributed%20to%20Bartonella.
Mental Health Disorders Associated with Bartonella spp. and Toxoplasma gondii, accessed August 18, 2025, https://jebms.org/full-text/124
Depressiveness and Neuroticism in Bartonella Seropositive and Seronegative Subjects—Preregistered Case-Controls Study - Frontiers, accessed August 18, 2025, https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2018.00314/full
Have a Heart or Broken Heart: Bartonella and Cardiovascular Diseases - National Veterinary Laboratory, accessed August 18, 2025, https://www.natvetlab.com/PDF/newsletters/Vol-7-No-2.pdf
In the Literature—November 1, 2021 | Clinical Infectious Diseases | Oxford Academic, accessed August 18, 2025, https://academic.oup.com/cid/article/73/9/i/6418531
Bartonellosis - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7152345/
Cutaneous manifestations of bartonellosis - SciELO, accessed August 18, 2025, https://www.scielo.br/j/abd/a/XpFVC7nXDd9mCxNqdtZQhPg/?lang=en
Bartonellosis | Lyme Disease, accessed August 18, 2025, https://www.columbia-lyme.org/bartonellosis
emedicine.medscape.com, accessed August 18, 2025, https://emedicine.medscape.com/article/213169-clinical#:~:text=Associated%20symptoms%20include%20joint%20and,aching%2C%20and%20occasionally%20with%20hyperexcitability.
Bartonella spp. Bacteremia and Rheumatic Symptoms in Patients from Lyme Disease–endemic Region - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3358077/
Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) and the Urgent Need to Identify Diagnostic Biomarkers and Risk Factors - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11418572/
Clinical Overview of Long COVID - CDC, accessed August 18, 2025, https://www.cdc.gov/long-covid/hcp/clinical-overview/index.html
Long COVID Basics - CDC, accessed August 18, 2025, https://www.cdc.gov/long-covid/about/index.html
www.cdc.gov, accessed August 18, 2025, https://www.cdc.gov/long-covid/about/index.html#:~:text=Long%20COVID%20is%20a%20serious,years%20after%20COVID%2D19%20illness.
Long COVID Clinical Guidance - CDC, accessed August 18, 2025, https://www.cdc.gov/long-covid/hcp/clinical-guidance/index.html
Long COVID (Post-COVID Conditions, PCC) - Yale Medicine, accessed August 18, 2025, https://www.yalemedicine.org/conditions/long-covid-post-covid-conditions-pcc
CDC Science and the Public Health Approach to Long COVID, accessed August 18, 2025, https://www.cdc.gov/long-covid/php/scientific-approach/index.html
Long COVID: major findings, mechanisms and recommendations - PubMed, accessed August 18, 2025, https://pubmed.ncbi.nlm.nih.gov/36639608/
1 In 8 COVID-19 Patients Develop Long-Term Symptoms: Lancet Study | News, accessed August 18, 2025, https://swachhindia.ndtv.com/1-in-8-covid-19-patients-develop-long-term-symptoms-lancet-study-69934/
What Can Chronic Lyme Disease Teach Us About Long Covid? - Psychology Today, accessed August 18, 2025, https://www.psychologytoday.com/us/blog/recovery-lyme/202201/what-can-chronic-lyme-disease-teach-us-about-long-covid
Long COVID: Post-COVID Conditions, Symptoms & Treatment - Cleveland Clinic, accessed August 18, 2025, https://my.clevelandclinic.org/health/diseases/25111-long-covid
Scientists unlock causes of post-COVID chronic fatigue - Biocentaur, accessed August 18, 2025, https://biocentaur.com/scientists-unlock-causes-of-post-covid-chronic-fatigue/
Overview of Long COVID: Navigating the Aftermath - Journal of Brown Hospital Medicine, accessed August 18, 2025, https://bhm.scholasticahq.com/article/133879-overview-of-long-covid-navigating-the-aftermath
What is Long COVID, accessed August 18, 2025, https://recovercovid.org/long-covid
Lancet study says long Covid has more than 200 symptoms | Coronavirus update | Latest English News - YouTube, accessed August 18, 2025,
Long COVID: Lasting effects of COVID-19 - Mayo Clinic, accessed August 18, 2025, https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-long-term-effects/art-20490351
Long COVID-19 Pathophysiology: What Do We Know So Far? - MDPI, accessed August 18, 2025, https://www.mdpi.com/2076-2607/11/10/2458
Pathophysiology of Long COVID 1: An unbalanced immune system - Altea Network, accessed August 18, 2025, https://altea-network.com/en/blog/145-pathophysiology-immune
Pathophysiological, immunological, and inflammatory features of long COVID - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10932978/
From Long Covid to Long Lyme: Persistent Infections Drive Chronic Illness, accessed August 18, 2025, https://www.bayarealyme.org/blog/from-long-covid-to-long-lyme-persistent-infections-drive-chronic-illness/
COVID-19 and Immune Dysregulation, a Summary and Resource - WHN, accessed August 18, 2025, https://whn.global/covid-19-and-immune-dysregulation-a-summary-and-resource/
Immune dysregulation and system pathology in COVID-19 - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7993139/
Full article: Immune dysregulation and system pathology in COVID-19, accessed August 18, 2025, https://www.tandfonline.com/doi/full/10.1080/21505594.2021.1898790
Paradigm of immune dysregulation in coronavirus disease-2019 infection, accessed August 18, 2025, https://www.explorationpub.com/Journals/ei/Article/1003126
Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response - MDPI, accessed August 18, 2025, https://www.mdpi.com/2075-1729/13/11/2121
A review of cytokine-based pathophysiology of Long COVID symptoms - Frontiers, accessed August 18, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1011936/full
Role of Endothelium in Cardiovascular Sequelae of Long COVID - MDPI, accessed August 18, 2025, https://www.mdpi.com/2227-9059/11/8/2239
Long Covid and the Vascular Endothelium - Global Virus Network, accessed August 18, 2025, https://gvn.org/long-covid-and-the-vascular-endothelium/
Damage to endothelial barriers and its contribution to long COVID - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10134732/
The Significance of Endothelial Dysfunction in Long COVID-19 for the Possible Future Pandemic of Chronic Kidney Disease and Cardiovascular Disease - MDPI, accessed August 18, 2025, https://www.mdpi.com/2218-273X/14/8/965
Impact of tick-borne/vector-borne infection on post-COVID symptoms, accessed August 18, 2025, https://polybio.org/projects/impact-of-tick-borne-vector-borne-infection-on-post-covid-symptoms/
Viral Reactivation and Long COVID - RTHM, accessed August 18, 2025, https://rthm.com/articles/viral-reactivation-and-long-covid/
Lights and Shadows of Long COVID: Are Latent Infections the Real Hidden Enemy? - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11641947/
COVID isn't just infecting you—it could be reactivating viruses that have been dormant in your body for years - UNMC, accessed August 18, 2025, https://www.unmc.edu/healthsecurity/transmission/2023/04/04/covid-isnt-just-infecting-you-it-could-be-reactivating-viruses-that-have-been-dormant-in-your-body-for-years-2/
COVID-19 Can Reactivate Latent Viruses | Immunology - Labroots, accessed August 18, 2025, https://www.labroots.com/trending/immunology/24041/covid-19-reactivate-latent-viruses
SARS-CoV-2 infection triggers reactivation of latent viruses in people with chronic fatigue syndrome - News-Medical.net, accessed August 18, 2025, https://www.news-medical.net/news/20221116/SARS-CoV-2-infection-triggers-reactivation-of-latent-viruses-in-people-with-chronic-fatigue-syndrome.aspx
Long COVID Signs and Symptoms - CDC, accessed August 18, 2025, https://www.cdc.gov/long-covid/signs-symptoms/index.html
Lessons from Long Haul Lyme & Long Haul COVID | Johns Hopkins Rheumatology, accessed August 18, 2025,
Long Lyme/Co-Infections and Long COVID | What do they have in common? - YouTube, accessed August 18, 2025,
Treatment strategies for long-haul COVID and persistent Lyme disease - LymeDisease.org, accessed August 18, 2025, https://www.lymedisease.org/long-haul-covid-lyme-disease/
Neuropsychiatric Implications by Brian Fallon, MD, MPH - Lyme Summit 2023 - YouTube, accessed August 18, 2025,
Postacute Sequelae of COVID (PASC or Long COVID): An Evidenced-Based Approach - PMC - National Institutes of Health (NIH) |, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11363684/
Long-term effects of COVID-19 (long COVID) - NHS, accessed August 18, 2025, https://www.nhs.uk/conditions/covid-19/long-term-effects-of-covid-19-long-covid/
Post-COVID Care: A Neurologist's Perspective - Mount Sinai, accessed August 18, 2025, https://reports.mountsinai.org/article/neuro2021-05-neurology-post-covid-19-care
Long COVID: neurological manifestations - an updated narrative review - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10901563/
Neuropsychiatric Manifestations of Long COVID-19: A Narrative Review of Clinical Aspects and Therapeutic Approaches - MDPI, accessed August 18, 2025, https://www.mdpi.com/2075-1729/15/3/439
Heart Problems After Covid-19 - Cleveland Clinic, accessed August 18, 2025, https://my.clevelandclinic.org/health/articles/heart-problems-after-covid
Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9524329/
Long COVID May Cause Long-Term Changes in the Heart ... - Mount Sinai, accessed August 18, 2025, https://www.mountsinai.org/about/newsroom/2025/long-covid-may-cause-long-term-changes-in-the-heart-and-lungs-and-may-lead-to-cardiac-and-pulmonary-diseases
Covid infection increases the risk of heart attack and stroke in women: Here's why, accessed August 18, 2025, https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/covid-infection-increases-the-risk-of-heart-attack-and-stroke-in-women-heres-why/articleshow/123355014.cms
New-onset chronic musculoskeletal pain in Long COVID | JPR - Dove Medical Press, accessed August 18, 2025, https://www.dovepress.com/clinical-characterization-of-new-onset-chronic-musculoskeletal-pain-in-peer-reviewed-fulltext-article-JPR
pmc.ncbi.nlm.nih.gov, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9212788/#:~:text=Musculoskeletal%20(MSK)%20pain%20is%20being,characteristics%2C%20and%20underlying%20pathophysiological%20mechanisms.
Musculoskeletal and Neuropathic Pain in COVID-19 - MDPI, accessed August 18, 2025, https://www.mdpi.com/2075-4418/14/3/332
Neuropathic component of chronic musculoskeletal pain in patients with post-COVID-19: A cross-sectional study - Archives of Rheumatology, accessed August 18, 2025, https://archivesofrheumatology.org/full-text/1608
Understanding the long-term interplay of SARS-CoV-2 immune and inflammatory responses with proteases in COVID-19 recovery: a longitudinal study - Frontiers, accessed August 18, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1517933/full
Shared inflammatory pathways and therapeutic strategies in COVID-19 and cancer immunotherapy - PMC - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8126446/
Inflammatory pathways in patients with post-acute sequelae of COVID-19: The role of the clinical immunologist, accessed August 18, 2025, https://recovercovid.org/publications/inflammatory-pathways-patients-post-acute-sequelae-covid-19-role-clinical-immunologist
Unmasking Bartonella henselae infection in the shadows of long COVID thanks to clinical metagenomics - PubMed, accessed August 18, 2025, https://pubmed.ncbi.nlm.nih.gov/38472519/
Unmasking Bartonella henselae infection in the shadows of long COVID thanks to clinical metagenomics | Request PDF - ResearchGate, accessed August 18, 2025, https://www.researchgate.net/publication/378904752_Unmasking_Bartonella_henselae_infection_in_the_shadows_of_long_COVID_thanks_to_clinical_metagenomics
Unmasking Bartonella henselae infection in the shadows of long COVID thanks to clinical metagenomics - The Commonwealth Scientific and Industrial Research Organisation (CSIRO) - Discovery, accessed August 18, 2025, https://discovery.csiro.au/discovery/fulldisplay?docid=cdi_hal_primary_oai_HAL_hal_04562918v1&context=PC&vid=61CSIRO_INST:CSIRO&lang=en&search_scope=All&adaptor=Primo%20Central&tab=All&query=creator%2Cexact%2C%20Ber%C3%A7ot%2C%20B%C3%A9atrice%20%2CAND&facet=creator%2Cexact%2C%20Ber%C3%A7ot%2C%20B%C3%A9atrice%20&mode=advanced&offset=0
Unmasking Bartonella henselae infection in the shadows of long COVID thanks to clinical metagenomics - Aspire PANS, accessed August 18, 2025, https://aspire.care/research/unmasking-bartonella-henselae-infection-in-the-shadows-of-long-covid-thanks-to-clinical-metagenomics/
(PDF) Post-COVID reactivation of latent Bartonella henselae ..., accessed August 18, 2025, https://www.researchgate.net/publication/380002652_Post-COVID_reactivation_of_latent_Bartonella_henselae_infection_a_case_report_and_literature_review
Post-COVID reactivation of latent Bartonella henselae infection: a case report and literature review | springermedizin.de, accessed August 18, 2025, https://www.springermedizin.de/post-covid-reactivation-of-latent-bartonella-henselae-infection-/27003446
Post-COVID reactivation of latent Bartonella henselae infection: a case report and literature review - PubMed, accessed August 18, 2025, https://pubmed.ncbi.nlm.nih.gov/38649899/
Post-COVID reactivation of latent Bartonella henselae infection: a case report and literature review - PubMed Central, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11034019/
A case of Bartonellosis presenting as a puzzling multisystem disorder complicated by nosocomial COVID-19 infection, accessed August 18, 2025, https://casereports.bmj.com/content/14/8/e244002
Case study: Lyme disease in patient with Long COVID - Daniel Cameron MD, accessed August 18, 2025, https://danielcameronmd.com/case-study-lyme-disease-in-patient-with-long-covid/
Bartonella Seroreactivity Among Persons Experiencing ..., accessed August 18, 2025, https://academic.oup.com/ofid/article/8/6/ofab230/6272404
Diagnosis of Cat Scratch Disease with Detection of Bartonella henselae by PCR: a Study of Patients with Lymph Node Enlargement - ASM Journals, accessed August 18, 2025, https://journals.asm.org/doi/10.1128/jcm.43.8.3800-3806.2005
Leading Off: Bartonellosis: Clinical and diagnostic challenges - DVM360, accessed August 18, 2025, https://www.dvm360.com/view/leading-bartonellosis-clinical-and-diagnostic-challenges
A Retrospective Analysis of Systemic Bartonella henselae Infection in Children - MDPI, accessed August 18, 2025, https://www.mdpi.com/2076-2607/12/4/666
Bartonella Infection | Choose the Right Test - ARUP Consult, accessed August 18, 2025, https://arupconsult.com/content/bartonella-species
Bartonella spp. Infections Identified by Molecular Methods, United States - PMC, accessed August 18, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9973681/
Navigating diagnostic challenges in Bartonella-induced infective endocarditis: a case report, accessed August 18, 2025, https://pubmed.ncbi.nlm.nih.gov/40016798/
The Consequences of Doxycycline Postexposure Prophylaxis - Contagion Live, accessed August 18, 2025, https://www.contagionlive.com/view/the-consequences-of-doxycycline-postexposure-prophylaxis
The Escalating Challenges of Gram-Resistant Organisms: The Role of Cefiderocol, accessed August 18, 2025, https://www.contagionlive.com/view/the-escalating-challenges-of-gram-resistant-organisms-the-role-of-cefiderocol
COVID-19 Long haulers Syndrome: Treatment, Symptoms and the Relation to Chronic Lyme Disease. - Stram Center for Integrative Medicine, accessed August 18, 2025, https://stramcenter.com/blog/blog-detail/covid-19-long-haulers-syndrome-treatment-symptoms-and-the-relation-to-chronic-lyme-disease/
Long COVID: Similar to Lyme disease and ME/CFS? - Dr. Todd Maderis, accessed August 18, 2025, https://drtoddmaderis.com/long-covid-similiar-to-lyme-disease-and-me-cfs
NIH-funded study finds Long COVID affects adolescents differently than younger children, accessed August 18, 2025, https://recovercovid.org/news/nih-funded-study-finds-long-covid-affects-adolescents-differently-younger-children
NIH invites public participation to inform future Long COVID clinical trials, accessed August 18, 2025, https://recovercovid.org/news/nih-invites-public-participation-inform-future-long-covid-clinical-trials
Long COVID | NHLBI, NIH, accessed August 18, 2025, https://www.nhlbi.nih.gov/covid/long-covid
LongCovid Research Consortium (LCRC) - PolyBio Research Foundation, accessed August 18, 2025, https://polybio.org/longcovid/
Two Years Later: Bruce Patterson, Long COVID, ME/CFS/FM and the Poll - Health Rising, accessed August 18, 2025, https://www.healthrising.org/blog/2023/03/21/patterson-long-covid-chronic-fatigue-lyme-poll/
Long COVID Research| NSU Institute for Neuro-Immune Medicine, accessed August 18, 2025, https://www.nova.edu/nim/research-studies/long-covid.html
long covid Archives - Bay Area Lyme Foundation, accessed August 18, 2025, https://www.bayarealyme.org/tag/long-covid/
New study highlights scale and impact of long COVID, accessed August 18, 2025, https://healthsciences.arizona.edu/news/releases/new-study-highlights-scale-and-impact-long-covid