The Multisystem Pathophysiology of SARS-CoV-2 Infection
COVID-19 is a systemic disease driven by ACE2 disruption, cytokine storms, and endotheliitis—causing multi-organ damage and laying the groundwork for Long COVID.
Introduction
Coronavirus Disease 2019 (COVID-19), caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), emerged as a primary respiratory illness but has since been unmasked as a formidable systemic disease.1 The initial paradigm of COVID-19 as a viral pneumonia, while accurate in describing its most common severe manifestation, fails to capture the profound and widespread impact the virus exerts across the human body. A more complete understanding frames COVID-19 as a systemic disease of the endothelium, initiated by a respiratory pathogen but propagated by a complex and often devastating interplay between direct viral cytotoxicity, profound dysregulation of host homeostatic systems, and a hyperinflammatory immune response.3 The clinical spectrum of the disease—ranging from asymptomatic infection to multi-organ failure—is a direct reflection of this systemic pathophysiology.1
This chapter will systematically deconstruct the cascade of events that underpins the multisystem nature of COVID-19. It begins at the molecular level, examining the precise mechanisms of viral entry and the immediate consequences for cellular function. It then explores the host's immunological reaction, detailing how a protective response can escalate into a destructive "cytokine storm." Finally, it applies these foundational principles to provide a comprehensive, system-by-system analysis of the pathophysiology, clinical manifestations, and long-term sequelae of SARS-CoV-2 infection. By tracing the pathogenic journey from a single virion's interaction with a cell surface receptor to the systemic chaos of critical illness, this overview will establish the essential framework for understanding the full clinical scope of this pandemic-defining disease.
Section 1: The Molecular Gateway: Viral Entry and Host Cell Interaction
The capacity of SARS-CoV-2 to induce a systemic disease is fundamentally rooted in the molecular mechanisms it exploits to invade host cells. These initial events not only determine the virus's tropism and pathogenicity but also trigger a cascade of host responses that drive the disease process. The interaction between the viral Spike protein and the host's Angiotensin-Converting Enzyme 2 (ACE2) receptor is the critical initiating step that defines the virus's cellular targets and sets in motion a profound disruption of physiological homeostasis.
1.1 The SARS-CoV-2 Spike Protein and its Affinity for ACE2
The SARS-CoV-2 virion is an enveloped, positive-sense, single-stranded RNA virus distinguished by the prominent glycoprotein projections on its surface, which create a crown-like, or "corona," appearance.6 These projections are the Spike (S) glycoproteins, trimeric structures that are essential for mediating host cell attachment and membrane fusion.6 The S protein is composed of two functional subunits: the S1 subunit, which contains the Receptor-Binding Domain (RBD), and the S2 subunit, which facilitates membrane fusion.7
The primary functional receptor for SARS-CoV-2 is Angiotensin-Converting Enzyme 2 (ACE2), a type I transmembrane protein that serves as the principal "doorway" for the virus into human cells.8 The RBD within the S1 subunit has been shown to have an exceptionally high binding affinity for human ACE2.7 This affinity is estimated to be 10 to 20 times greater than that of the S protein from the original SARS-CoV, a key molecular feature that is thought to contribute significantly to the high transmissibility and infectivity of SARS-CoV-2.7
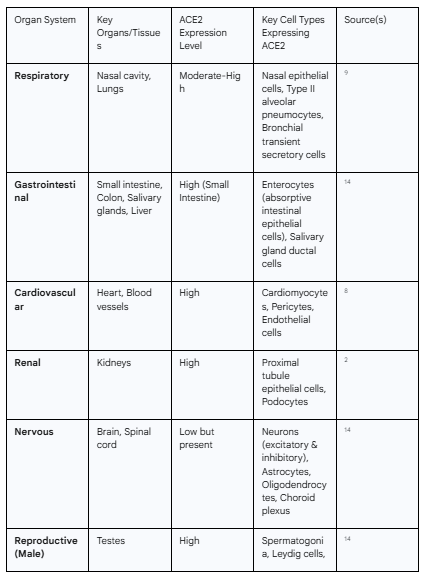
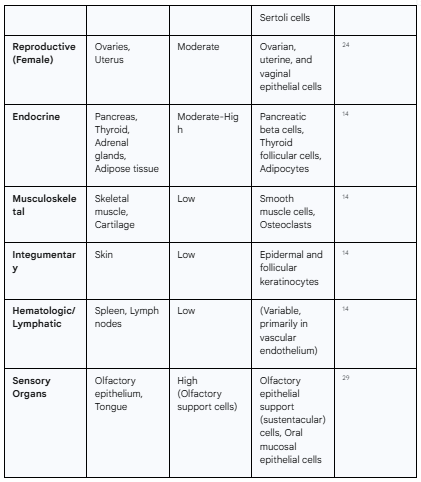
The widespread physiological distribution of the ACE2 receptor is the primary determinant of the virus's organ tropism. While often considered a respiratory virus, the multi-organ nature of COVID-19 is a direct consequence of this pre-existing map of vulnerabilities across the human body. Analyses of large-scale tissue expression databases, such as the Genotype-Tissue Expression (GTEx) project and The Cancer Genome Atlas (TCGA), have revealed that ACE2 is expressed in a multitude of organs beyond the lungs.9 High levels of ACE2 expression are found in the enterocytes of the small intestine, the proximal tubule cells of the kidneys, the heart, the testes, and the vascular endothelium.9 Moderate to low expression is also detected in the brain, salivary glands, adipose tissue, and thyroid.14 This distribution provides the molecular basis for the diverse clinical manifestations of COVID-19, including diarrhea, acute kidney injury, myocarditis, and neurological symptoms, as the virus can directly infect cells in these disparate locations.1
1.2 The Role of TMPRSS2 and Other Host Proteases in Viral Priming
While binding of the S protein to ACE2 is a necessary first step for viral entry, it is not sufficient. The process requires proteolytic "priming" of the S protein by host cell proteases to unlock its fusogenic potential.11 The most critical of these is Transmembrane Serine Protease 2 (TMPRSS2), a cell surface protease that is often co-expressed with ACE2 in susceptible tissues.9
Upon S protein binding to ACE2, TMPRSS2 cleaves the S protein at two key sites: the boundary between the S1 and S2 subunits (S1/S2 site) and the S2' site within the S2 subunit.8 This cleavage event is a crucial activation step. It triggers irreversible conformational changes in the S2 subunit, leading to the exposure of a fusion peptide that inserts into the host cell membrane.8 The S2 subunit then refolds into a stable post-fusion conformation, pulling the viral and host cell membranes together and facilitating their fusion. This process allows the viral RNA to be released into the host cell's cytoplasm, where it can hijack the cellular machinery for replication.16 The co-expression of both ACE2 and TMPRSS2 in a given cell type, such as bronchial transient secretory cells and type II alveolar pneumocytes, is therefore a strong predictor of its susceptibility to productive infection.9
A unique and significant feature of the SARS-CoV-2 S protein is the presence of a polybasic furin cleavage site at the S1/S2 boundary, a motif that is absent in SARS-CoV and related bat coronaviruses.7 Furin is a ubiquitous proprotein convertase found within the host cell's secretory pathway. This allows the S protein to be pre-cleaved, or "pre-activated," during virion assembly and egress from an infected cell. This pre-activation reduces the virus's dependence on target cell proteases like TMPRSS2 for entry, potentially broadening its cellular tropism and enhancing its infectivity and spread across different tissues.7
1.3 Consequences of Viral Binding: ACE2 Downregulation and RAAS Dysregulation
The interaction between SARS-CoV-2 and ACE2 inflicts damage through a mechanism of sabotage that extends far beyond direct viral replication. The binding of the virus to ACE2 and its subsequent internalization into the cell via endocytosis leads to a functional downregulation of ACE2 on the cell surface.12 This event has profound pathophysiological consequences because ACE2 is a critical negative regulator of the Renin-Angiotensin-Aldosterone System (RAAS), a hormonal cascade essential for regulating blood pressure, fluid balance, and inflammation.9
The classical RAAS pathway involves the conversion of Angiotensin I (Ang I) to Angiotensin II (Ang II) by Angiotensin-Converting Enzyme (ACE). Ang II is a potent peptide hormone that, upon binding to its type 1 receptor (AT1R), mediates vasoconstriction, sodium and water retention, and promotes inflammation, fibrosis, and thrombosis.3 ACE2 provides a crucial counter-regulatory axis to this system. It functions as a carboxypeptidase that degrades the pro-inflammatory Ang II into the heptapeptide Angiotensin (1-7).3 Ang (1-7) then acts on the Mas receptor to exert opposing effects: vasodilation, anti-inflammation, anti-fibrosis, and anti-thrombosis.3
The SARS-CoV-2-mediated downregulation of ACE2 disrupts this delicate homeostatic balance, tipping the scales in favor of the classical ACE/Ang II/AT1R axis.20 The loss of ACE2 function leads to an accumulation of Ang II and a concurrent depletion of the protective Ang (1-7).3 This unopposed Ang II activity creates a systemic environment characterized by vasoconstriction, increased vascular permeability, and a potent pro-inflammatory and pro-thrombotic state.3 This RAAS dysregulation is not a secondary effect but a primary driver of pathology, contributing significantly to the severe lung injury, acute respiratory distress syndrome (ARDS), myocarditis, and systemic endothelial dysfunction that characterize severe COVID-19.12 In this way, the virus weaponizes a vital host homeostatic system, turning it against the body and amplifying the damage initiated by the infection itself. This explains why the severity of organ damage can often appear disproportionate to the measured viral load in some patients; the virus triggers a self-sustaining cascade of host-driven injury.
Table 1.1: Distribution and Expression Levels of ACE2 in Human Organ Systems
Section 2: The Host Response: Immunity, Inflammation, and Systemic Consequences
While viral entry mechanisms dictate which tissues are vulnerable, it is the host's immune response that largely determines the clinical course and severity of COVID-19. In most individuals, the immune system successfully contains and clears the virus, resulting in mild or asymptomatic disease.5 In a subset of patients, however, this response becomes dysregulated and hyperinflammatory, driving the most severe and life-threatening manifestations of the illness. This section details the progression from a standard antiviral response to a pathological state of systemic inflammation, endotheliitis, and coagulopathy.
2.1 Innate and Adaptive Immune Activation
Following infection of respiratory epithelial cells, viral components such as double-stranded RNA are detected by host pattern recognition receptors (PRRs), including Toll-like receptors (TLRs).3 This recognition triggers the innate immune response, a critical first line of defense characterized by the production of antiviral cytokines, most notably Type I and Type III interferons (IFNs).32 A timely and robust IFN response is crucial for limiting early viral replication and spread.32
However, a key pathogenic feature of SARS-CoV-2 is its array of viral proteins that can antagonize and delay this initial IFN response.32 By evading early immune detection, the virus is able to replicate relatively unchecked in the initial days of infection, leading to a higher viral burden.5 This delay is followed by a later, but often more robust and dysregulated, inflammatory response. This response involves the recruitment and activation of innate immune cells, including monocytes, macrophages, and neutrophils, to the site of infection.35 These cells, in turn, release a battery of pro-inflammatory cytokines and chemokines that orchestrate the subsequent activation of the adaptive immune system, which comprises T and B lymphocytes responsible for targeted viral clearance and the generation of long-term immunological memory.35
2.2 The Cytokine Storm: Pathogenesis and Systemic Impact
In patients who develop severe COVID-19, the immune response escalates into an excessive and uncontrolled hyperinflammatory state known as a Cytokine Storm or Cytokine Release Syndrome (CRS).34 This phenomenon is not a failure of the immune system but rather an over-exuberant and dysregulated activation cascade that becomes detrimental to the host.34 It is characterized by the massive and sustained release of pro-inflammatory cytokines and chemokines into the circulation from a large number of activated immune cells.34
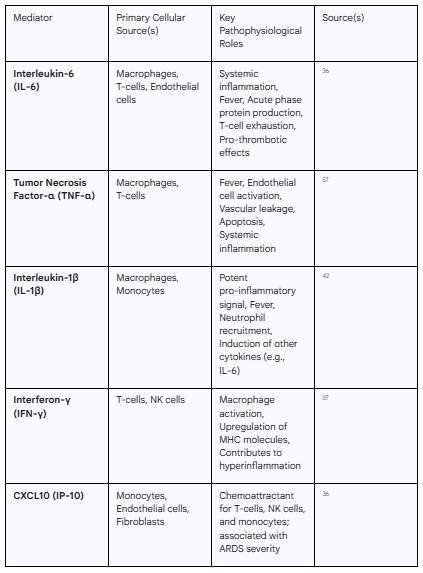
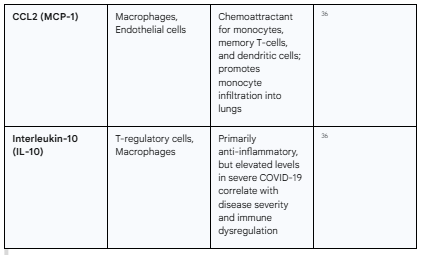
The cytokine profile of severe COVID-19 is complex, but key mediators have been consistently identified. These include Interleukin-6 (IL-6), Interleukin-1β (IL-1β), Tumor Necrosis Factor-alpha (TNF-α), Interferon-gamma (IFN-γ), and various chemokines such as CXCL10 (IP-10) and CCL2 (MCP-1).36 High circulating levels of these molecules, particularly IL-6, are strongly correlated with disease severity, the need for mechanical ventilation, and mortality.36
This "storm" is a primary driver of the most critical complications of COVID-19. The flood of cytokines causes widespread systemic inflammation, fever, and constitutional symptoms.41 It leads to increased vascular permeability and leakage, resulting in tissue edema and contributing directly to the pathogenesis of ARDS.36 The hyperinflammatory state also contributes to lymphocyte apoptosis, leading to the characteristic lymphopenia observed in severe cases, and can ultimately culminate in shock, multi-organ failure, and death.3 The pathogenesis is further amplified by mitochondrial dysfunction induced by the virus, which fuels inflammation, and by pyroptosis, a highly inflammatory form of programmed cell death that releases additional damage-associated molecular patterns (DAMPs) into the tissue environment, perpetuating the inflammatory cycle.32
Table 2.1: Key Cytokines and Chemokines in COVID-19-Associated Cytokine Release Syndrome
2.3 Endotheliitis and COVID-19-Associated Coagulopathy (CAC)
A cardinal and defining feature of severe COVID-19 is widespread endothelial cell injury and dysfunction, a condition termed endotheliitis.4 The vascular endothelium, a single layer of cells lining all blood vessels, is a critical regulator of vascular tone, permeability, inflammation, and coagulation. It is also a key battleground in COVID-19. The endothelium is a target for both direct viral infection, due to its expression of ACE2, and indirect injury from the circulating mediators of the cytokine storm.4
This endothelial injury has catastrophic consequences. It disrupts the integrity of the vascular barrier, leading to fluid leakage into tissues and contributing to organ edema, particularly in the lungs.35 It promotes a pro-inflammatory state by upregulating adhesion molecules that facilitate the recruitment of immune cells into tissues.4 Most critically, endothelial dysfunction triggers a profound and life-threatening hypercoagulable state known as COVID-19-Associated Coagulopathy (CAC).4
CAC is a unique thrombo-inflammatory condition characterized by markedly elevated levels of D-dimer (a fibrin degradation product) and fibrinogen, significant endothelial cell activation, and platelet hyperreactivity.4 This creates a potent pro-thrombotic milieu that leads to the formation of both macro-thromboses (e.g., deep vein thrombosis, pulmonary embolism) and, perhaps more insidiously, widespread micro-thromboses within the small vessels of various organs.4 This thrombotic tendency is a major contributor to mortality and morbidity in COVID-19, directly causing life-threatening events like pulmonary embolism, stroke, and myocardial infarction, and contributing to organ dysfunction through microvascular occlusion and ischemia.4
The most devastating outcomes of COVID-19 arise not from a single mechanism but from the catastrophic convergence of the three core pathologies discussed: RAAS dysregulation, the cytokine storm, and endotheliitis. These are not independent processes but form a self-amplifying, vicious triad. The loss of ACE2 function drives RAAS imbalance, leading to an excess of pro-inflammatory and pro-thrombotic Ang II.3 This Ang II-driven inflammation directly contributes to and amplifies the cytokine storm.21 The cytokines from the storm, along with the direct effects of Ang II, then converge to inflict widespread damage on the vascular endothelium.4 This endothelial injury, in turn, precipitates the hypercoagulable state of CAC. The resulting microthrombi cause ischemic damage to organs, which releases more inflammatory signals, further fueling the cytokine storm and creating a destructive positive feedback loop. This integrated pathology explains the clinical pivot of COVID-19 from a primarily respiratory illness to a systemic vascular and thrombotic disease, and it underscores why therapies targeting inflammation (e.g., corticosteroids) and coagulation (e.g., anticoagulants) became cornerstones in the management of severe cases.
Section 3: A System-by-System Analysis of SARS-CoV-2 Pathophysiology
The foundational principles of viral entry, RAAS dysregulation, cytokine-mediated inflammation, and endotheliitis provide a unified framework for understanding the diverse clinical manifestations of COVID-19. This section will apply these core mechanisms to explain the specific pathophysiology, clinical presentations, and long-term sequelae of SARS-CoV-2 infection in each major organ system.
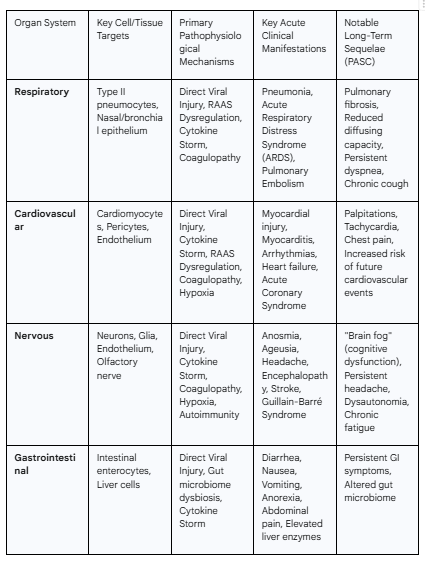
3.1 The Respiratory System: The Primary Battlefield
Pathophysiology
The respiratory tract serves as the principal portal of entry for SARS-CoV-2 and is the primary site of its most severe pathology.2 Upon inhalation, the virus binds to ACE2 receptors, which are highly expressed on nasal epithelial cells and, critically, on type II alveolar pneumocytes deep within the lungs.9 Viral replication within these cells leads to direct cytopathic effects, cellular injury, and a form of inflammatory cell death known as pyroptosis.18 This process releases viral particles to infect adjacent cells and also liberates damage-associated molecular patterns (DAMPs) and pro-inflammatory cytokines, initiating a localized inflammatory response.18
As the infection progresses, this localized inflammation, amplified by the systemic cytokine storm and local RAAS dysregulation, leads to profound damage to the alveolar-capillary barrier.55 Endothelial and epithelial cell death increases vascular permeability, allowing protein-rich edema fluid to flood the alveolar spaces.35 This accumulation of fluid, along with cellular debris and fibrin, leads to the classic histological pattern of diffuse alveolar damage (DAD), characterized by the formation of hyaline membranes lining the alveoli, which severely impairs gas exchange.55
Clinical Manifestations
The clinical spectrum of respiratory involvement is broad, ranging from mild, self-limiting upper respiratory symptoms like cough and sore throat to severe, life-threatening viral pneumonia.2 In the most severe cases, this progresses to Acute Respiratory Distress Syndrome (ARDS), a form of hypoxemic respiratory failure that often requires mechanical ventilation.52 COVID-19-associated ARDS exhibits several unique features. Patients may present with profound hypoxemia that is disproportionate to the degree of respiratory distress or radiological findings, a phenomenon termed "silent hypoxemia".47 Furthermore, a significant component of the pathology is vascular. Widespread microthrombosis in the pulmonary capillaries and a high incidence of pulmonary embolism contribute significantly to the ventilation-perfusion (V/Q) mismatch and dead space ventilation, exacerbating hypoxemia.48
Long-Term Sequelae
A substantial proportion of individuals who survive severe COVID-19 pneumonia experience long-term respiratory complications, which are a core component of Post-Acute Sequelae of COVID-19 (PASC), or Long COVID.60 The extensive alveolar damage and subsequent dysregulated healing process can lead to the development of pulmonary fibrosis, a condition of permanent lung scarring that results in stiff lungs and chronic respiratory impairment.2 Survivors often exhibit persistent symptoms such as dyspnea (shortness of breath), chronic cough, and reduced exercise tolerance.60 Pulmonary function tests frequently reveal a restrictive ventilatory defect (reduced lung volumes) and, most commonly, an impaired diffusing capacity for carbon monoxide (DLCO), reflecting damage to the alveolar-capillary membrane.48
3.2 The Cardiovascular System: Direct and Indirect Insults
Pathophysiology
The cardiovascular system is a major target of both direct viral effects and indirect systemic insults in COVID-19. ACE2 is highly expressed in the heart, specifically on cardiomyocytes, cardiac fibroblasts, and pericytes, creating a pathway for direct viral invasion.12 This can lead to direct viral-induced cell death and inflammation of the heart muscle, or myocarditis.57
However, indirect mechanisms of injury are more common and often more significant drivers of cardiovascular complications.67 These include:
Systemic Inflammation: The cytokine storm can cause profound myocardial depression and dysfunction, even without direct viral presence in the heart tissue.57
Hypoxia: Severe respiratory failure leads to systemic hypoxemia, placing immense stress on the heart muscle and creating a mismatch between oxygen supply and demand, which can result in myocardial injury (Type 2 myocardial infarction).68
RAAS Dysregulation: The imbalance of the RAAS, with an excess of Ang II, promotes vasoconstriction, inflammation, and fibrosis within the myocardium.3
Endotheliitis and Thrombosis: Widespread inflammation and injury to the coronary endothelium can destabilize atherosclerotic plaques or promote de novo thrombus formation, leading to acute coronary syndromes (ACS) and myocardial infarction.4 Microvascular thrombosis can also cause diffuse myocardial damage.65
Clinical Manifestations
The clinical presentations of cardiovascular involvement are diverse. Acute myocardial injury, evidenced by elevated cardiac troponin levels, is common in hospitalized patients and is a strong predictor of mortality.53 Other manifestations include myocarditis, pericarditis (inflammation of the sac surrounding the heart), new-onset or exacerbated heart failure, life-threatening arrhythmias (such as atrial fibrillation and ventricular tachycardia), and Takotsubo (stress) cardiomyopathy.53 Thromboembolic events, including myocardial infarction, stroke, and pulmonary embolism, are also frequent and severe complications.53
Long-Term Sequelae
Cardiovascular symptoms are prominent in Long COVID. Many individuals report persistent palpitations, chest pain, tachycardia (fast heart rate), and dyspnea on exertion for months after the acute infection.65 There is significant concern that the inflammatory and fibrotic damage incurred during the acute phase may lead to an increased long-term risk of developing chronic conditions such as heart failure, arrhythmias, and other cardiovascular events.65
3.3 The Nervous System: A Neurotropic Threat
Pathophysiology
SARS-CoV-2 can affect both the central nervous system (CNS) and the peripheral nervous system (PNS) through a variety of mechanisms.71 While ACE2 expression in the brain is generally low, it is present in key locations that may serve as entry points or sites of injury.14 These include neurons and glial cells, the vascular endothelium, and strategically important areas like the choroid plexus (which produces cerebrospinal fluid) and certain brainstem nuclei.15
Potential routes of CNS invasion include:
Retrograde Neuronal Transport: The virus may travel along the olfactory nerve from the infected nasal epithelium, through the cribriform plate, and into the brain—a hypothesis supported by the high incidence of anosmia.15
Hematogenous Spread: The virus can travel through the bloodstream and cross a compromised blood-brain barrier (BBB), particularly in the context of severe systemic inflammation that increases BBB permeability.72
Infection of Endothelial Cells: Direct infection of the ACE2-expressing endothelial cells of the cerebral vasculature can cause local inflammation and thrombosis.72
Beyond direct viral neuro-invasion, indirect neurological injury is a major contributor to pathology. This can result from systemic hypoxia due to respiratory failure, cytokine-mediated neuroinflammation crossing the BBB, cerebrovascular events (ischemic and hemorrhagic stroke) stemming from CAC, metabolic derangements, and post-infectious autoimmune phenomena that target nervous system structures.71
Clinical Manifestations
A wide spectrum of neurological complications has been documented. Common, non-specific symptoms include headache, dizziness, myalgia, and fatigue.71 More severe CNS manifestations include encephalopathy (diffuse brain dysfunction causing altered mental status), encephalitis (inflammation of the brain parenchyma), meningitis (inflammation of the meninges), acute cerebrovascular disease, and seizures.75 In the PNS, aside from the characteristic loss of smell and taste, post-infectious autoimmune conditions like Guillain-Barré syndrome (GBS) and acute disseminated encephalomyelitis (ADEM) have been reported.71
Long-Term Sequelae
Persistent neurological and neuropsychiatric symptoms are a defining and debilitating feature of Long COVID. The most frequently reported symptom is "brain fog," a constellation of cognitive complaints including poor concentration, memory deficits, slowed thinking, and mental fatigue.78 The pathophysiology of brain fog is an area of intense research, with leading hypotheses including persistent low-grade neuroinflammation, a chronically "leaky" blood-brain barrier allowing inflammatory molecules to enter the CNS, direct viral persistence in tissues such as the brainstem, and altered neuronal metabolism.73
3.4 The Gastrointestinal System and Microbiome: A Secondary Reservoir
Pathophysiology
The gastrointestinal (GI) tract is a major target for SARS-CoV-2, with the enterocytes of the small intestine exhibiting some of the highest levels of ACE2 expression in the human body.14 This allows for efficient viral infection and replication within the intestinal lining, leading to direct mucosal injury, inflammation, and increased intestinal permeability ("leaky gut").82 This active replication results in high levels of viral shedding in feces for a prolonged period, even after respiratory samples become negative, which has implications for potential fecal-oral transmission.82
Furthermore, SARS-CoV-2 infection profoundly disrupts the gut microbiome, the complex community of microorganisms residing in the gut. This disruption, known as dysbiosis, is characterized by a decrease in the abundance of beneficial, immunomodulatory bacteria (such as Faecalibacterium prausnitzii and Bifidobacterium species) and a concurrent enrichment of opportunistic pathogens.87 This imbalance is significant because a healthy microbiome is crucial for regulating the host immune system. Gut dysbiosis can exacerbate local and systemic inflammation through the "gut-lung axis," a bidirectional communication pathway, potentially contributing to the severity of respiratory disease and the systemic cytokine storm.86 This dysbiotic state has been shown to persist even after viral clearance.90
Clinical Manifestations
GI symptoms are common in COVID-19 and can sometimes be the presenting complaint. The most frequent manifestations include anorexia (loss of appetite), nausea, vomiting, diarrhea, and abdominal pain.1 Liver injury, typically presenting as a mild to moderate elevation of transaminase enzymes (ALT and AST), is also frequently observed. The mechanism of liver injury is likely multifactorial, stemming from a combination of direct viral effects on liver cells, systemic inflammation, hypoxia-induced ischemic injury, and drug-induced hepatotoxicity from medications used to treat COVID-19.18
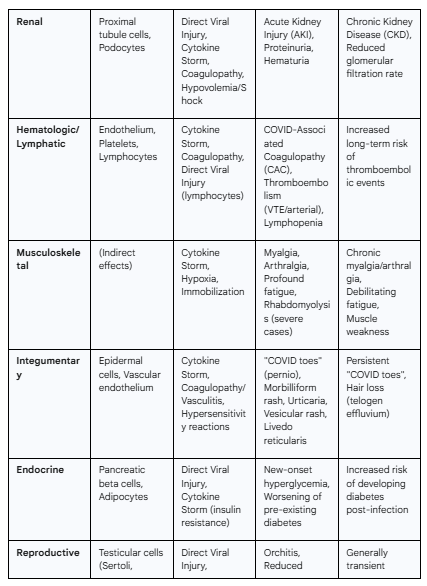
3.5 The Renal System: A Target of Systemic Insult
Pathophysiology
The kidneys are highly susceptible to injury during COVID-19. They exhibit high expression of ACE2 and TMPRSS2, particularly in the epithelial cells of the proximal tubules and in podocytes, which are critical cells of the glomerulus, making them vulnerable to direct viral infection and cytopathic effects.2 Autopsy studies have confirmed the presence of the virus in kidney tissue.
However, the development of Acute Kidney Injury (AKI) in COVID-19 is often the result of a convergence of multiple pathogenic mechanisms.94 In addition to direct viral injury, these include:
Systemic Inflammation: The cytokine storm can induce acute tubular necrosis through direct inflammatory damage.2
Endotheliitis and Thrombosis: Widespread microthrombi can form in the glomerular and peritubular capillaries, leading to ischemic kidney injury.95
Hemodynamic Instability: In critically ill patients, shock and hypovolemia can lead to decreased renal perfusion and ischemic AKI.2
RAAS Dysregulation: The viral-induced imbalance of the RAAS can alter renal hemodynamics and promote inflammation and fibrosis within the kidney.2
Rhabdomyolysis: In some cases, breakdown of skeletal muscle can release myoglobin, which is nephrotoxic.95
Clinical Manifestations
The most significant renal complication of COVID-19 is AKI, which is observed in a substantial proportion of hospitalized patients and is a strong independent predictor of mortality.95 Other common clinical findings include proteinuria (protein in the urine) and hematuria (blood in the urine), reflecting glomerular and tubular damage. In severe cases, AKI can progress to the point of requiring renal replacement therapy, such as dialysis.95
3.6 The Hematologic and Lymphatic Systems: The Coagulopathy Conundrum
Pathophysiology
The hematologic and lymphatic systems are at the epicenter of the pathophysiology of severe COVID-19. The most prominent feature is the development of COVID-19-Associated Coagulopathy (CAC), a unique thrombo-inflammatory state driven by the convergence of profound endothelial injury, platelet activation, and a massive inflammatory response that activates the coagulation cascade.4 This state is distinct from classic disseminated intravascular coagulation (DIC). In its early stages, CAC is typically characterized by markedly elevated D-dimer and fibrinogen levels, with relatively normal platelet counts and only mildly prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT).49 This profile reflects a state of hypercoagulation rather than the consumptive coagulopathy seen in typical DIC.49
The lymphatic system is also significantly affected. The characteristic lymphopenia (a marked reduction in the number of circulating lymphocytes) seen in severe disease is a key laboratory finding and a strong predictor of poor outcomes.18 The mechanisms are thought to include direct viral infection of lymphocytes, cytokine-induced apoptosis, and potential sequestration of lymphocytes in inflamed tissues. Autopsy studies have also shown evidence of viral replication in lymph nodes and significant pathological changes, including splenic atrophy, which further contributes to immune dysregulation.21
Clinical Manifestations
The primary clinical manifestation of CAC is a profound hypercoagulable state, leading to exceptionally high rates of both venous and arterial thrombosis.4 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and life-threatening pulmonary embolism (PE), is particularly common, especially in critically ill patients.96 Arterial thrombotic events, such as ischemic stroke and myocardial infarction, also occur at an increased rate.4 While thrombosis is the dominant feature, bleeding can also occur, particularly in critically ill patients who may progress to a more consumptive phase of coagulopathy later in their disease course.96
3.7 The Musculoskeletal System: The Burden of Myalgia and Fatigue
Pathophysiology
Musculoskeletal symptoms are among the most frequently reported manifestations of COVID-19, both in the acute phase and during long-term recovery.27 While direct viral invasion of muscle tissue is possible, as ACE2 expression is present in smooth muscle, the primary driver of these symptoms is believed to be the systemic inflammatory response.27 Pro-inflammatory cytokines, such as IL-6 and TNF-α, which are released in large quantities during the cytokine storm, are known to be potent inducers of myalgia (muscle pain) and arthralgia (joint pain).97 Other contributing factors may include hypoxia in severe disease, direct damage to the endothelium of muscle vasculature, and the catabolic effects of prolonged immobilization and critical illness, which lead to muscle wasting and weakness.27
Clinical Manifestations
The most common musculoskeletal symptoms during acute COVID-19 are myalgia, arthralgia, and profound fatigue.27 The pain can be localized or generalized and varies in severity. In more severe cases, significant muscle injury can lead to rhabdomyolysis, a condition characterized by the breakdown of muscle tissue and the release of damaging proteins into the blood, which can be identified by markedly elevated creatine kinase (CK) levels.99
Long-Term Sequelae
Persistent myalgia, arthralgia, and debilitating fatigue are core features of Long COVID.70 This chronic pain and exhaustion can significantly impair physical function, mental health, and overall quality of life.105 The underlying mechanisms are thought to involve persistent low-grade inflammation, potential autoimmune processes, and changes in the central nervous system's processing of pain signals (central sensitization).105
3.8 The Integumentary System: Cutaneous Clues to Infection
Pathophysiology
The skin serves as a visible indicator of the systemic nature of COVID-19, with a wide variety of cutaneous manifestations reported. The presence of ACE2 receptors in epidermal and follicular cells of the skin suggests a potential for direct viral effects.28 However, the majority of skin changes are thought to be secondary to the systemic processes occurring during the infection.106 These mechanisms include hypersensitivity or exanthematous reactions to the virus, direct effects of the cytokine storm on the skin, and, most notably, vascular phenomena such as vasculitis (inflammation of blood vessels) or microthrombosis in the cutaneous vessels.106
Clinical Manifestations
A diverse array of rashes has been associated with COVID-19. These include morbilliform (measles-like) and erythematopapular eruptions, urticaria (hives), and vesicular (chickenpox-like) rashes.28 Vascular-related skin changes include livedo reticularis (a lace-like, purplish discoloration) and purpuric or vasculitic rashes.28
Perhaps the most specific and widely discussed cutaneous finding is pernio-like acral lesions, colloquially known as "COVID toes".108 This condition presents as erythematous to violaceous (red to purplish), swollen, and sometimes painful or itchy papules and patches on the toes and, less commonly, the fingers.109 It often appears in younger patients with otherwise mild or asymptomatic infections and is thought to be related to a robust interferon response or microvascular injury.106
Long-Term Sequelae
Some cutaneous manifestations can persist for weeks to months and are considered part of Long COVID. The most common of these are prolonged cases of "COVID toes" and telogen effluvium, a form of diffuse, non-scarring hair loss that typically occurs several months after a significant physiological stressor like a severe infection.102
3.9 The Endocrine System: Metabolic Dysregulation
Pathophysiology
The endocrine system is a significant target in COVID-19, with implications for metabolic homeostasis. ACE2 is expressed in several key endocrine glands, including the pancreatic islets (specifically beta cells), the thyroid, and the adrenal glands.14 The pancreas is of particular concern. Evidence from in vitro and autopsy studies suggests that SARS-CoV-2 can directly infect and damage pancreatic beta cells, the sole producers of insulin in the body.26 This direct cytopathic effect can impair or destroy the cells' ability to secrete insulin.
Concurrently, the severe systemic inflammatory state induced by the cytokine storm causes profound peripheral insulin resistance, meaning that tissues like muscle and fat become less responsive to insulin's effects.26 The combination of impaired insulin secretion and increased insulin resistance creates a "perfect storm" for metabolic dysregulation, leading to hyperglycemia.
Clinical Manifestations
The most significant endocrine manifestation of COVID-19 is new-onset hyperglycemia or the severe worsening of pre-existing diabetes.26 This is a common finding in hospitalized patients and is strongly associated with increased disease severity and mortality. It is important to note that individuals with pre-existing diabetes are themselves at a much higher risk of developing severe COVID-19, creating a dangerous bidirectional relationship between the virus and this metabolic condition.1 Other endocrine dysfunctions, such as subacute thyroiditis, have also been reported but are less common.
3.10 The Reproductive System: A Question of Long-Term Impact
Pathophysiology
Both the male and female reproductive systems express the necessary machinery for SARS-CoV-2 entry, raising concerns about the virus's impact on fertility and reproductive health.24 The male testes exhibit particularly high co-expression of ACE2 and TMPRSS2, especially in spermatogonia and Leydig and Sertoli cells, making them a highly plausible target for direct viral infection.14 Autopsy studies of men who died from COVID-19 have provided evidence of orchitis (inflammation of the testes), infiltration of immune cells, germ cell destruction, and damage to Leydig cells (which produce testosterone) and Sertoli cells (which support sperm development).23 In addition to direct viral effects, the systemic inflammation and high fever associated with severe COVID-19 can independently and temporarily impair spermatogenesis and testosterone production.23
In the female reproductive system, ACE2 is expressed in the ovaries, uterus, and vagina, though at lower levels than in the testes.24 The primary mechanism of impact appears to be indirect. The systemic inflammatory response and significant psychological stress of the illness can disrupt the delicate hormonal balance of the hypothalamic-pituitary-ovarian (HPO) axis, which regulates the menstrual cycle.115 During pregnancy, severe maternal COVID-19 is associated with placental pathology and an increased risk of adverse outcomes, primarily driven by maternal systemic disease rather than direct fetal infection, as vertical transmission is rare.24
Clinical Manifestations
In males, acute infection can be associated with testicular pain (orchitis), documented reductions in serum testosterone levels, and transiently impaired semen parameters, including decreased sperm count, motility, and ejaculate volume.23 In females, a common report is transient menstrual cycle irregularities, such as changes in cycle length (prolonged cycles) and menstrual volume (decreased volume).25 Pregnant women with severe COVID-19 face increased risks of pre-term birth and other complications.24
Long-Term Sequelae
The current evidence suggests that most of the observed effects on reproductive function are transient. Studies following men after recovery have generally shown that semen parameters and testosterone levels tend to improve and return to baseline within 3 to 6 months.23 Similarly, menstrual irregularities in women typically resolve within a few cycles post-infection.115 However, the potential for long-term impacts, particularly in men who experienced severe orchitis, remains an area of ongoing investigation.
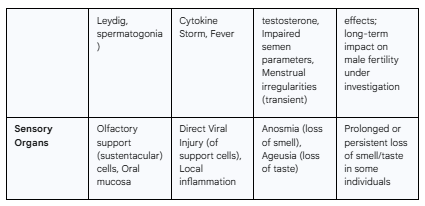
3.11 The Sensory Organs: The Telltale Signs of Anosmia and Ageusia
Pathophysiology
The sudden loss of the sense of smell (anosmia) and taste (ageusia or dysgeusia) emerged as highly specific and common early symptoms of COVID-19.30 The underlying mechanism for anosmia has been a subject of intense study. It does not appear to be caused by direct viral infection of the olfactory sensory neurons themselves, as these cells express very little or no ACE2.31 Instead, research has shown that the virus robustly infects the surrounding non-neuronal cells in the olfactory epithelium, particularly the support (sustentacular) cells and basal stem cells, which are rich in ACE2 and TMPRSS2.29 The infection and subsequent inflammation of these vital support cells are thought to disrupt the structural and metabolic environment necessary for the proper function of the olfactory neurons, leading to a rapid and profound, but often temporary, loss of smell.31
The mechanism for the loss of taste is less well-defined but is thought to follow a similar principle. ACE2 is expressed in various cells of the oral mucosa and tongue, and their infection could disrupt the function of taste receptor cells indirectly.29 Alterations in saliva composition due to infection of the salivary glands may also play a role.29
Clinical Manifestations
Anosmia and ageusia are hallmark symptoms of COVID-19. Their onset is often sudden and can occur in the absence of nasal congestion, distinguishing it from the smell loss associated with a common cold.31 Frequently, these sensory deficits are among the first symptoms to appear, sometimes preceding fever and cough, and can even be the sole manifestation of the infection in otherwise asymptomatic individuals.118 While recovery of smell and taste is typical, usually occurring within a few weeks, the deficit can be prolonged for months in a subset of individuals, significantly impacting their quality of life.29
Table 3.1: Summary of SARS-CoV-2 Effects Across Organ Systems
Conclusion: An Integrated View of a Systemic Disease
This chapter has systematically delineated the transformation of COVID-19 from what was initially perceived as a localized respiratory infection into a complex, multisystem disease of profound consequence. The pathogenic journey, from the high-affinity molecular interaction at the ACE2 receptor to the systemic chaos of a cytokine storm and endotheliitis, reveals a pathology defined by interconnectedness. The evidence demonstrates that the dysregulation of the Renin-Angiotensin-Aldosterone System, the hyperinflammatory host immune response, and the profound disruption of the vascular endothelium do not act in isolation. Instead, they form a destructive triad that drives injury across nearly every organ system, providing a unified explanation for the remarkably diverse clinical manifestations of the disease.
Understanding these integrated pathways is paramount for both clinicians managing patients and researchers developing therapeutic strategies. The acute, multisystem insult detailed herein also lays the biological foundation for the chronic and often debilitating condition known as Post-Acute Sequelae of COVID-19 (PASC), or Long COVID. This lingering legacy of the pandemic, characterized by persistent symptoms affecting a multitude of organ systems, underscores the virus's far-reaching and lasting impact on human health. The principles outlined in this chapter serve as the essential groundwork upon which the more specialized discussions of organ-specific pathology, clinical management, and long-term outcomes in the subsequent chapters of this volume will be built.
Acknowledgement
I acknowledge the use of Gemini AI in the preparation of this report. Specifically, it was used to: (1) brainstorm and refine the initial research questions; (2) assist in writing and debugging Python scripts for statistical analysis; and (3) help draft, paraphrase, and proofread sections of the final manuscript. I reviewed, edited, and assume full responsibility for all content.
Works cited
Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2 - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2020.594495/full
New insights into the pathogenesis of SARS-CoV-2 during and after the COVID-19 pandemic - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1363572/full
Hyperinflammatory Response in COVID-19: A Systematic Review - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9962879/
Full article: Nuts and bolts of COVID-19 associated coagulopathy: the essentials for management and treatment - Taylor & Francis Online, accessed August 31, 2025, https://www.tandfonline.com/doi/full/10.1080/00325481.2021.1974212
Pathophysiology and clinical management of coronavirus disease (COVID-19): a mini-review - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1116131/full
Structural understanding of SARS-CoV-2 virus entry to host cells - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10683510/
Cell entry mechanisms of SARS-CoV-2 - PNAS, accessed August 31, 2025, https://www.pnas.org/doi/10.1073/pnas.2003138117
The possible mechanism and research progress of ACE2 involved in cardiovascular injury caused by COVID-19: a review - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2024.1409723/full
SARS-CoV-2, ACE2 expression, and systemic organ invasion ..., accessed August 31, 2025, https://journals.physiology.org/doi/abs/10.1152/physiolgenomics.00087.2020
Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8236094/
pmc.ncbi.nlm.nih.gov, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10683510/#:~:text=The%20current%20understanding%20of%20the,%2C%202020%3B%20Peng%20et%20al.
Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System | Circulation Research - American Heart Association Journals, accessed August 31, 2025, https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.120.317015
Expression pattern and function of SARS-CoV-2 receptor ACE2 | Biosafety and Health, accessed August 31, 2025, https://mednexus.org/doi/10.1016/j.bsheal.2021.08.003
Analysis of 2019-nCoV receptor ACE2 expression in different ..., accessed August 31, 2025, https://atm.amegroups.org/article/view/49436/html
The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ..., accessed August 31, 2025, https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2020.573095/full
Animation of SARS-CoV-2 entry into human host-cell. - YouTube, accessed August 31, 2025,
Host interactions with SARS-CoV-2 - Xiong Laboratory - Yale University, accessed August 31, 2025, https://xiong.yale.edu/research/host-interactions-sars-cov-2
COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment - Oxford Academic, accessed August 31, 2025, https://academic.oup.com/pmj/article/97/1147/312/6969661
ACE2 as a Therapeutic Target for COVID-19; Its Role in Infectious Processes and Regulation by Modulators of the RAAS System - MDPI, accessed August 31, 2025, https://www.mdpi.com/2077-0383/9/7/2096
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19 - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8727358/
Lethal COVID-19 associates with RAAS-induced inflammation for multiple organ damage including mediastinal lymph nodes | PNAS, accessed August 31, 2025, https://www.pnas.org/doi/10.1073/pnas.2401968121
The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain | bioRxiv, accessed August 31, 2025, https://www.biorxiv.org/content/10.1101/2020.04.07.030650v3
SARS-CoV-2 Infection and the Male Reproductive System: A Brief Review - MDPI, accessed August 31, 2025, https://www.mdpi.com/2075-1729/13/2/586
SARS-CoV-2, COVID-19, and Reproduction: Effects on Fertility, Pregnancy, and Neonatal Life - MDPI, accessed August 31, 2025, https://www.mdpi.com/2227-9059/10/8/1775
SARS-CoV-2 infection and COVID-19 and human reproduction – A changing perspective – A 2022 update | Clinics - Elsevier, accessed August 31, 2025, https://www.elsevier.es/en-revista-clinics-22-articulo-sars-cov-2-infection-covid-19-human-reproduction-S1807593223000029
Understanding the interplay between COVID-19 and diabetes: insights for the post-pandemic era - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2025.1599969/full
Musculoskeletal involvement: COVID-19 and post COVID 19 - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10186015/
COVID-19 Rashes: Pictures, Symptoms, and Treatment - Health, accessed August 31, 2025, https://www.health.com/condition/infectious-diseases/coronavirus/types-of-rashes-covid-19
Potential pathogenesis of ageusia and anosmia in COVID‐19 patients - Fonovim, accessed August 31, 2025, https://www.fonovim.com.br/arquivos/ff7a14aec99d2d2fad658c8be380dc33-Potential-pathogenesis-of-ageusia-and-anosmia-in-COVID-19-patients.pdf
Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7488171/
How COVID-19 Causes Loss of Smell | Harvard Medical School, accessed August 31, 2025, https://hms.harvard.edu/news/how-covid-19-causes-loss-smell
Hyperinflammatory Immune Response and COVID-19: A Double Edged Sword - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.742941/full
A review of cytokine-based pathophysiology of Long COVID symptoms - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1011936/full
SARS-CoV-2 causes a different cytokine response compared to other cytokine storm-causing respiratory viruses in severely ill patients | medRxiv, accessed August 31, 2025, https://www.medrxiv.org/content/10.1101/2020.11.14.20231878v1.full-text
COVID-19 induced ARDS: immunopathology and therapeutics, accessed August 31, 2025, https://www.explorationpub.com/Journals/ei/Article/1003101
Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.01708/full
Cytokine Storm in COVID-19: Immunopathogenesis and Therapy - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8876409/
Pathogenesis and treatment of cytokine storm in COVID-19 - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8573840/
The pathogenesis and treatment of the "Cytokine Storm" in COVID-19 - SEPSIS-one.org, accessed August 31, 2025, https://sepsis-one.org/en/the-pathogenesis-and-treatment-of-the-cytokine-storm-in-covid-19/
Novel COVID-19 (SARS-CoV-2) Infection Induced Cytokine Storm - Dove Medical Press, accessed August 31, 2025, https://www.dovepress.com/attenuating-the-effects-of-novel-covid-19-sars-cov-2-infection-induced-peer-reviewed-fulltext-article-JIR
Pathogenesis and Treatment of Cytokine Storm Induced by Infectious Diseases - MDPI, accessed August 31, 2025, https://www.mdpi.com/1422-0067/22/23/13009
Full article: Comparison of the Characteristics of Cytokine Storm and Immune Response Induced by SARS-CoV, MERS-CoV, and SARS-CoV-2 Infections - Taylor & Francis Online, accessed August 31, 2025, https://www.tandfonline.com/doi/full/10.2147/JIR.S329697
Cytokine storm in COVID-19 pathway - Abcam, accessed August 31, 2025, https://www.abcam.com/en-us/technical-resources/pathways/cytokine-storm-in-covid-19-pathway
What is a Cytokine Storm? - News-Medical, accessed August 31, 2025, https://www.news-medical.net/health/What-is-Cytokine-Storm.aspx
Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.589095/full
Inflammation and organ damage in severe COVID-19 tied to mitochondrial dysfunction, accessed August 31, 2025, https://www.news-medical.net/news/20241203/Inflammation-and-organ-damage-in-severe-COVID-19-tied-to-mitochondrial-dysfunction.aspx
Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: Similarities and Differences - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2022.829771/full
Pathophysiology of pulmonary function anomalies in COVID-19 survivors - ERS Publications, accessed August 31, 2025, https://publications.ersnet.org/content/breathe/17/3/210065
Coagulopathy in COVID-19: Manifestations and management, accessed August 31, 2025, https://www.ccjm.org/content/87/8/461
COVID‐19‐related coagulopathy: A review of pathophysiology and pharmaceutical management - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8239905/
Hematologic disorders associated with COVID-19: a review - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7789889/
COVID-19 and Respiratory System Disorders | Arteriosclerosis, Thrombosis, and Vascular Biology - AHA Journals, accessed August 31, 2025, https://www.ahajournals.org/doi/10.1161/ATVBAHA.120.314515
COVID-19-Associated Cardiovascular Complications - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8293160/
COVID-19 Lung Damage: Symptoms, Scarring & Conditions - Cleveland Clinic, accessed August 31, 2025, https://my.clevelandclinic.org/health/diseases/covid-lung
Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8162817/
COVID-19 Lung Injury: Unique and Familiar Aspects of Pathophysiology - MDPI, accessed August 31, 2025, https://www.mdpi.com/2076-3417/14/23/11048
SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19 - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7833750/
Mechanism of COVID-19 Causing ARDS: Exploring the Possibility of Preventing and Treating SARS-CoV-2 - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2022.931061/full
Is COVID-19 different from other causes of acute respiratory distress syndrome? - MedNexus, accessed August 31, 2025, https://mednexus.org/doi/10.1016/j.jointm.2023.02.003
COVID-19: long-term respiratory consequences - SciELO, accessed August 31, 2025, https://www.scielo.br/j/spmj/a/VHR5DZBxcb6Gh5z5wjWb36Q/
Post-COVID syndrome: pulmonary complications - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8771021/
Long COVID Basics - CDC, accessed August 31, 2025, https://www.cdc.gov/long-covid/about/index.html
Long-term effects of COVID-19 (long COVID) - NHS, accessed August 31, 2025, https://www.nhs.uk/conditions/covid-19/long-term-effects-of-covid-19-long-covid/
Respiratory Complications after COVID-19 - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8907756/
Heart Problems After Covid-19 - Cleveland Clinic, accessed August 31, 2025, https://my.clevelandclinic.org/health/articles/heart-problems-after-covid
Understanding Myocarditis After COVID-19 - Summa Health, accessed August 31, 2025, https://www.summahealth.org/flourish/entries/2025/05/understanding-myocarditis-after-covid-19
Cardiovascular Complications of COVID-19 Disease: A Narrative Review - MDPI, accessed August 31, 2025, https://www.mdpi.com/2079-9721/13/8/252
COVID's Damage Lingers in the Heart | Harvard Medicine Magazine, accessed August 31, 2025, https://magazine.hms.harvard.edu/articles/covids-damage-lingers-heart
Myocarditis - Symptoms and causes - Mayo Clinic, accessed August 31, 2025, https://www.mayoclinic.org/diseases-conditions/myocarditis/symptoms-causes/syc-20352539
Long COVID Information | Mount Sinai - New York, accessed August 31, 2025, https://www.mountsinai.org/health-library/diseases-conditions/long-covid
Neurological complications in patients with SARS-CoV-2 infection: a systematic review, accessed August 31, 2025, https://www.scielo.br/j/anp/a/mKwsJ5vjCBZZF8Xywpvmvcs/
review on pathogenesis of nervous system SARS-CoV–2 damage - NEUROLOGY OF COVID–19 - NCBI Bookshelf, accessed August 31, 2025, https://www.ncbi.nlm.nih.gov/books/NBK579780/
Researchers identify mechanism behind brain fog in long COVID - CIDRAP, accessed August 31, 2025, https://www.cidrap.umn.edu/covid-19/researchers-identify-mechanism-behind-brain-fog-long-covid
Neurological complications in patients with SARS-CoV-2 infection: a systematic review - SciELO, accessed August 31, 2025, https://www.scielo.br/j/anp/a/mKwsJ5vjCBZZF8Xywpvmvcs/?format=pdf&lang=en
Neurological Complications of COVID-19: Underlying Mechanisms and Management - MDPI, accessed August 31, 2025, https://www.mdpi.com/1422-0067/22/8/4081
Complications and Pathophysiology of COVID-19 in the Nervous System - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2020.573421/full
Neurological manifestations of COVID-19: what do we know? - VJNeurology, accessed August 31, 2025, https://www.vjneurology.com/video/9e6-l1bkxr0-neurological-manifestations-of-covid-19-what-do-we-know/
Brain fog | Long-term effects of COVID-19 - NHS inform, accessed August 31, 2025, https://www.nhsinform.scot/long-term-effects-of-covid-19-long-covid/signs-and-symptoms/long-covid-brain-fog/
What doctors wish patients knew about long COVID-19 brain fog, accessed August 31, 2025, https://www.ama-assn.org/delivering-care/public-health/what-doctors-wish-patients-knew-about-long-covid-19-brain-fog
MSU, Corewell Health scientists find link between brain fog and long COVID, accessed August 31, 2025, https://humanmedicine.msu.edu/news/2025-MSU-and-Corewell-Health-scientists-find-link-between-brain-fog-and-long-COVID.html
Long Covid: Researchers find clues for "brain fog" origin - healthcare-in-europe.com, accessed August 31, 2025, https://healthcare-in-europe.com/en/news/long-covid-virus-persist-in-brainstem-after-infection.html
Full article: Gastrointestinal pathophysiology of SARS-CoV2 – a literature review, accessed August 31, 2025, https://www.tandfonline.com/doi/full/10.1080/20009666.2020.1811556
SARS-CoV-2 Variant-Specific Gastrointestinal Symptoms of COVID-19: 2023 Update - MDPI, accessed August 31, 2025, https://www.mdpi.com/2036-7422/14/4/32
Gastrointestinal Manifestations of SARS-CoV-2: Transmission, Pathogenesis, Immunomodulation, Microflora Dysbiosis, and Clinical Implications - MDPI, accessed August 31, 2025, https://www.mdpi.com/1999-4915/15/6/1231
COVID-19 and Gastrointestinal Tract: From Pathophysiology to Clinical Manifestations - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10608106/
The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19, accessed August 31, 2025, https://journals.physiology.org/doi/abs/10.1152/ajpgi.00148.2020
Gut Microbiota and COVID-19: Unraveling the Gut–Lung Axis and Immunomodulatory Therapies | ACS Infectious Diseases - ACS Publications, accessed August 31, 2025, https://pubs.acs.org/doi/10.1021/acsinfecdis.5c00250
Full article: Gut microbiota in COVID-19: new insights from inside, accessed August 31, 2025, https://www.tandfonline.com/doi/full/10.1080/19490976.2023.2201157
COVID-19 and the Human Gut Microbiome: An Under-Recognized Association - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9535107/
Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19 - The BMJ, accessed August 31, 2025, https://gut.bmj.com/content/70/4/698
COVID-19 disrupts gut microbiome - National Institutes of Health (NIH) |, accessed August 31, 2025, https://www.nih.gov/news-events/nih-research-matters/covid-19-disrupts-gut-microbiome
The Effect of COVID-19 on Gut Microbiota: Exploring the Complex Interplay and Implications for Human Health - MDPI, accessed August 31, 2025, https://www.mdpi.com/2624-5647/5/3/28
SARS-CoV-2 and gastrointestinal diseases - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2023.1177741/full
Pathogenesis of Acute Kidney Injury in Coronavirus Disease 2019 - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2021.586589/full
A review of Covid-19 and acute kidney injury: from pathophysiology to clinical results, accessed August 31, 2025, https://www.bjnephrology.org/en/article/a-review-of-covid-19-and-acute-kidney-injury-from-pathophysiology-toclinical-results/
COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection | Blood | American Society of Hematology, accessed August 31, 2025, https://ashpublications.org/blood/article/136/4/489/460672/COVID-19-and-coagulation-bleeding-and-thrombotic
A Review: The Manifestations, Mechanisms, and Treatments of Musculoskeletal Pain in Patients With COVID-19 - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/pain-research/articles/10.3389/fpain.2022.826160/full
Musculoskeletal Problems in Patients with COVID-19: A Review Study - Brieflands, accessed August 31, 2025, https://brieflands.com/articles/asjsm-111040
Musculoskeletal manifectations of the new coronavirus infection: focus on arthralgia and myalgia | Shostak | The Clinician, accessed August 31, 2025, https://klinitsist.abvpress.ru/Klin/article/view/472?locale=en_US
The Musculoskeletal Involvement After Mild to Moderate COVID-19 Infection - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2022.813924/full
Effects of COVID-19 on the Musculoskeletal System: Clinician's Guide - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8464590/
Signs and symptoms of long COVID - NHS inform, accessed August 31, 2025, https://www.nhsinform.scot/long-term-effects-of-covid-19-long-covid/about-long-covid/signs-and-symptoms-of-long-covid/
Long COVID Signs and Symptoms - CDC, accessed August 31, 2025, https://www.cdc.gov/long-covid/signs-symptoms/index.html
The Lesser-known Symptoms of Long Covid - Samitivej, accessed August 31, 2025, https://www.samitivejhospitals.com/article/detail/symptoms-of-long-covid
Clinical Characterization of New-Onset Chronic Musculoskeletal Pain in Long COVID: A Cross-Sectional Study - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11298172/
“COVID Toes” and Other Skin Conditions Tied to Coronavirus - Northwestern Medicine, accessed August 31, 2025, https://www.nm.org/-/media/northwestern/resources/nm-newsroom/covid%20toes%20and%20other%20skin%20conditions.pdf
COVID Toes and Other Rashes Associated with COVID-19 - Consult QD - Cleveland Clinic, accessed August 31, 2025, https://consultqd.clevelandclinic.org/covid-toes-and-other-rashes-associated-with-covid-19
Skin‐related symptoms found in people with COVID‐19 during the Delta and Omicron waves - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9878176/
COVID toes and other rashes COVID-19 may cause - American Academy of Dermatology, accessed August 31, 2025, https://www.aad.org/public/diseases/a-z/covid-toes
Covid Toe: Symptoms, Duration and Treatments | Ada Health, accessed August 31, 2025, https://ada.com/covid/covid-19-toes-symptoms/
Dermatological complications due to post‑COVID‑19 syndrome: A systematic review - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11619564/
SARS-CoV-2 effect on male infertility and its possible pathophysiological mechanisms, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8605861/
Updates in the pathophysiology of COVID-19 infection in male reproductive and sexual health: a literature review - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2023.1226858/full
Severe COVID could reduce male fertility - UGA Today, accessed August 31, 2025, https://news.uga.edu/covid-affects-male-fertility/
The impact of COVID-19 on women's reproductive system - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2024.1485022/full
COVID-19 reduces male fertility by affecting semen quality and hormone levels, accessed August 31, 2025, https://www.news-medical.net/news/20240911/COVID-19-reduces-male-fertility-by-affecting-semen-quality-and-hormone-levels.aspx
COVID-19 and male fertility: short- and long-term impacts of asymptomatic vs. symptomatic infection on male reproductive potential - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/reproductive-health/articles/10.3389/frph.2024.1403143/full
Anosmia and ageusia associated with coronavirus infection (COVID-19) - what is known?, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7401831/
Potential Mechanisms for COVID-19 Induced Anosmia and Dysgeusia - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2020.01039/full