SARS-CoV-2 Persistence in the Gastrointestinal Tract: A Unifying Pathophysiological Model for Systemic Long COVID
Persistent SARS-CoV-2 in the gut may drive Long COVID via inflammation, barrier damage, and microbiome disruption—making gut-targeted therapies a key treatment path.
Executive Summary
Post-Acute Sequelae of COVID-19 (PASC), or Long COVID, has emerged as a complex, multi-systemic chronic illness affecting millions worldwide. While its pathophysiology is multifaceted, a compelling body of evidence points to the gastrointestinal (GI) tract as a critical site for the persistence of SARS-CoV-2, acting as a viral reservoir that drives the chronic inflammatory state underlying a significant subset of Long COVID cases. This report synthesizes current virological, immunological, and clinical evidence to construct a unified model of gut-driven pathophysiology. The central thesis is that the high expression of angiotensin-converting enzyme 2 (ACE2) receptors renders the intestinal lining highly susceptible to infection, allowing for the establishment of a persistent viral reservoir long after respiratory clearance. This reservoir continually sheds viral antigens, initiating a self-perpetuating pathological cascade. This cascade involves profound alterations to the gut microbiome (dysbiosis), compromised intestinal barrier integrity ("leaky gut"), and the subsequent translocation of both viral components and microbial products into systemic circulation. This "two-hit" inflammatory stimulus fuels a chronic, low-grade systemic inflammatory response. This systemic inflammation, in turn, drives the diverse symptomatology of Long COVID, with the gut-brain axis serving as a primary conduit for translating gut-centric pathology into the debilitating neurological and autonomic symptoms, such as cognitive dysfunction ("brain fog") and dysautonomia, that are hallmarks of the condition. This model positions the gut reservoir not as one of many competing hypotheses but as a potential upstream driver that unifies other observed phenomena in Long COVID, including immune dysregulation, endothelial dysfunction, and neurotransmitter imbalances. Consequently, therapeutic strategies targeting the eradication of the viral reservoir, restoration of gut barrier function, and modulation of the microbiome represent promising avenues for the treatment of this debilitating syndrome.
1. Introduction: The Gastrointestinal Tract as a Primary Target and Reservoir for SARS-CoV-2
1.1. Biological Rationale: The Role of ACE2 Receptor Expression in Intestinal Tropism
The fundamental biological plausibility for the gastrointestinal tract serving as a major site of SARS-CoV-2 infection and persistence is anchored in its unique molecular landscape.1 The primary mechanism of SARS-CoV-2 cellular entry involves the binding of the viral spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells.3 While ACE2 is expressed in various tissues, the intestinal tract—specifically the epithelial cells of the ileum and colon—exhibits the highest density of ACE2 receptors in the entire human body, surpassing even that of the lungs.3 This exceptionally high receptor expression makes the gut a highly permissive environment for viral entry and subsequent replication.5
The process is further facilitated by the co-expression of cellular proteases, such as transmembrane protease serine 2 (TMPRSS2), which are essential for priming the viral S protein, a necessary step for the fusion of the viral and host cell membranes.4 The abundance of both ACE2 and TMPRSS2 on intestinal enterocytes establishes a robust mechanism for efficient viral infection of the gut lining.6
Beyond simply facilitating entry, the viral engagement of ACE2 has direct pathophysiological consequences that may precede a full-blown immune response. ACE2 plays a critical role in the local regulation of the renin-angiotensin system (RAS), where its primary enzymatic function is to convert the pro-inflammatory and vasoconstrictive peptide angiotensin II into the anti-inflammatory and vasodilatory peptide angiotensin (1-7).7 When SARS-CoV-2 binds to and effectively sequesters ACE2 receptors, this crucial enzymatic activity is diminished.4 This leads to a localized accumulation of angiotensin II within the gut wall, creating a pro-inflammatory, pro-thrombotic, and vasoconstrictive microenvironment.7 This disruption of local RAS homeostasis can be conceptualized as an initial, non-immunological "first hit" of inflammation, priming the gut for the subsequent cascade of immune activation, dysbiosis, and barrier dysfunction that characterizes the progression to Long COVID. Furthermore, this mechanism suggests that individual variations in baseline gut ACE2 expression or RAS regulation could be a significant predisposing factor for the development of GI-centric PASC.
1.2. From Acute Infection to Chronic Persistence: Establishing the Concept of a Viral Reservoir
While the acute phase of COVID-19 is often dominated by respiratory symptoms, a substantial body of evidence indicates that the involvement of the GI tract is not a transient phenomenon. One of the earliest and most consistent observations during the pandemic was the prolonged shedding of viral RNA in fecal samples, which often persisted for weeks or even months after nasopharyngeal swabs turned negative.5 Studies have documented that fecal samples can remain positive for SARS-CoV-2 RNA for nearly five weeks after respiratory clearance.5 This extended shedding pointed toward the gut as a site of prolonged viral activity.
This observation has led to the formulation of a central hypothesis for Long COVID: that for a subset of individuals, the condition is driven by the persistence of the virus or its components in tissue reservoirs, rather than being a purely "hit-and-run" post-viral syndrome characterized by a dysregulated but sterile immune state.2 A viral reservoir is defined as a specific anatomical site or cell type where a virus or its components (such as RNA or proteins) can endure long after the resolution of the acute, systemic infection.10 The gut, with its high permissiveness to infection and its unique immunological environment, has emerged as a leading candidate for such a reservoir in Long COVID.2 The concept posits that this persistent reservoir acts as a source of chronic antigenic stimulation, fueling the low-grade inflammation and immune dysregulation that are the hallmarks of PASC.10
2. Mechanisms of SARS-CoV-2 Persistence in the Intestinal Mucosa
2.1. Direct Evidence of a Persistent Viral Reservoir: Virological and Histopathological Findings
The hypothesis of a SARS-CoV-2 gut reservoir is supported by a growing body of direct evidence from virological and histopathological studies of human tissue. These findings move beyond the detection of inert viral fragments in stool to provide definitive proof of active and prolonged viral presence within the intestinal wall.
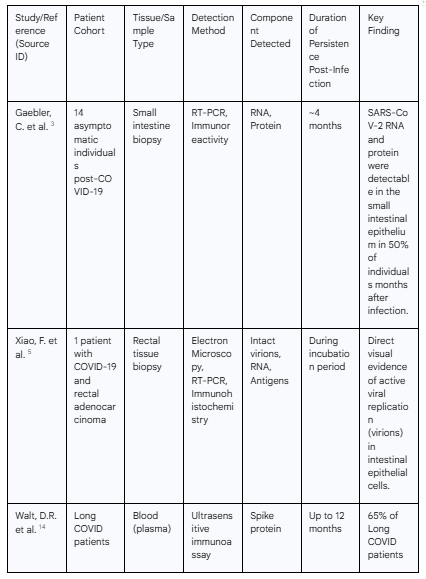
A landmark study provided the first direct evidence of active SARS-CoV-2 replication in the human intestine. Using electron microscopy on rectal tissue from a patient with COVID-19, researchers visualized intact, typical coronavirus virions within the cytoplasm of intestinal epithelial cells.5 This observation is of paramount importance, as it confirms that the gut can support the full viral life cycle, including the assembly of new, potentially infectious viral particles, rather than merely harboring residual, non-replicating viral RNA. The same study used immunohistochemistry to identify SARS-CoV-2 antigens on intestinal epithelial cells as well as on infiltrating lymphocytes and macrophages in the lamina propria, confirming the presence of viral proteins in multiple cell types within the gut.5
Multiple subsequent studies have corroborated the long-term persistence of viral components. An analysis of intestinal biopsies from asymptomatic individuals four months after their acute COVID-19 infection revealed the presence of SARS-CoV-2 RNA and immunoreactivity in the small intestines of 7 out of 14 individuals (50%).3 Another study focusing on a cohort of patients with inflammatory bowel disease (IBD) who had recovered from mild COVID-19 found detectable viral RNA or proteins in the gut tissue of 70% of participants seven months post-infection. Critically, roughly two-thirds of the individuals with detectable virus reported ongoing Long COVID symptoms like fatigue and memory issues, whereas none of those without detectable virus did, establishing a strong correlation between gut persistence and clinical symptomatology.14 More recent research has extended this timeline dramatically, with one team finding SARS-CoV-2 in colon tissue up to 676 days after the initial infection.15
Further compelling evidence comes from the detection of viral antigens in systemic circulation. A highly significant study detected SARS-CoV-2 proteins, most commonly the spike protein, in the blood of 65% of Long COVID patients up to 12 months after their initial diagnosis.14 Given the very short biological half-life of the spike protein in circulation, its sustained presence is a strong indicator of an active, persistent reservoir continuously shedding these proteins into the bloodstream.14 This finding provides a crucial link between a localized tissue reservoir and systemic pathology.
2.2. Immunological Sanctuaries and Viral Evasion Strategies
The ability of SARS-CoV-2 to establish a persistent reservoir in the gut is likely facilitated by both the unique immunological nature of the GI tract and the virus's own immune evasion capabilities. The gut is, in essence, a specialized "immunological sanctuary"—an anatomical site where immune responses are carefully modulated to maintain tolerance to a vast load of foreign antigens from food and the commensal microbiome.14 This state of controlled tolerance, which is essential for gut homeostasis, may inadvertently create a niche that is less hostile to viral persistence compared to more immunologically aggressive sites like the lungs. This concept is not unique to SARS-CoV-2; other viruses, such as Ebola, are known to persist in immune-privileged sites like the eyeball or testes, contributing to long-term post-viral symptoms.14
Furthermore, SARS-CoV-2 is not a passive bystander; it actively manipulates the host's immune defenses to its advantage. The virus possesses a sophisticated repertoire of non-structural and accessory proteins (e.g., NSP1, NSP6, NSP13, ORF6, ORF9b) that are known to antagonize the host's innate antiviral interferon (IFN) response.16 These viral proteins can block key steps in the IFN signaling cascade, such as inhibiting host mRNA translation, suppressing the phosphorylation of critical signaling molecules like IRF3 and TBK1, and blocking the nuclear import of transcription factors required to activate antiviral genes.16 By blunting this crucial first line of defense, the virus can replicate more efficiently in the initial stages of infection, likely facilitating the establishment of a stable reservoir in the gut before a robust and sterilizing adaptive immune response can be fully mounted.
This persistence of viral antigens in the gut is not a static phenomenon but appears to be an active driver of ongoing adaptive immune evolution. While systemic levels of antibodies like IgM and IgG tend to decay over the months following infection, studies have shown that memory B cells continue to evolve during this period, producing antibodies with progressively increased neutralizing breadth and potency.3 This process of affinity maturation requires continuous exposure to an antigen. The persistent viral reservoir in the gut likely provides this necessary stimulus, allowing the immune system in gut-associated lymphoid tissue (GALT) to continually "fine-tune" its antibody response.3 While this may be beneficial in generating more robust protection against reinfection, it is a double-edged sword. This same chronic antigenic stimulation that drives beneficial immune memory evolution is also the very mechanism hypothesized to fuel the persistent, low-grade inflammation that underlies the pathology of Long COVID.3 The specific nature of the resulting Long COVID phenotype may depend on factors such as which viral antigen persists (e.g., spike vs. nucleocapsid) and its precise location within the gut, which could dictate whether a T-cell-dominant or antibody-mediated pathology emerges.17
3. The Pathophysiological Cascade: From Viral Persistence to Systemic Inflammation
The establishment of a SARS-CoV-2 reservoir in the gut initiates a complex and self-perpetuating pathological cascade that culminates in chronic systemic inflammation. This process begins with local immune activation in the gut wall, which then drives profound changes in the microbiome and a physical breach of the intestinal barrier, ultimately allowing viral and microbial products to spill into the bloodstream and trigger a body-wide inflammatory response.
3.1. Persistent Antigen Shedding and Local Immune Activation
The first step in this cascade is the direct consequence of viral persistence: a chronic, localized inflammatory response within the gut wall. The continued presence of SARS-CoV-2 in intestinal epithelial cells and resident immune cells, such as macrophages, serves as a constant trigger for the gut-associated lymphoid tissue (GALT).5 Histopathological examination of gut biopsies from individuals with persistent virus reveals a characteristic infiltration of immune cells, primarily lymphocytes and macrophages, into the lamina propria, the layer of connective tissue just beneath the intestinal epithelium.5
This local immune battle is not confined to the general gut lining. It extends to specialized lymphoid structures within and associated with the gut. Research suggests that lymphoid organs like the tonsils and gut-associated lymphoid aggregates can harbor viral RNA for extended periods, leading to pronounced and sustained activation of tissue-resident memory (TRM) CD8+ T cells.17 These TRM cells act as sentinels within tissues, and their chronic activation in response to persistent antigens could be a major source of local pro-inflammatory cytokine production, contributing significantly to the state of chronic, low-grade inflammation that perturbs the gut microenvironment.17 This localized immune activity is a critical initiating event that sets the stage for the subsequent breakdown of gut homeostasis.
3.2. SARS-CoV-2-Induced Gut Dysbiosis: A Pro-inflammatory Shift in the Microbiome
The chronic inflammation and direct effects of viral replication profoundly disrupt the delicate balance of the gut microbiome, a condition known as dysbiosis.1 This is not a random alteration but a consistent and pro-inflammatory shift in the microbial community. Studies comparing the gut microbiomes of individuals with and without Long COVID have revealed a distinct signature associated with the condition.
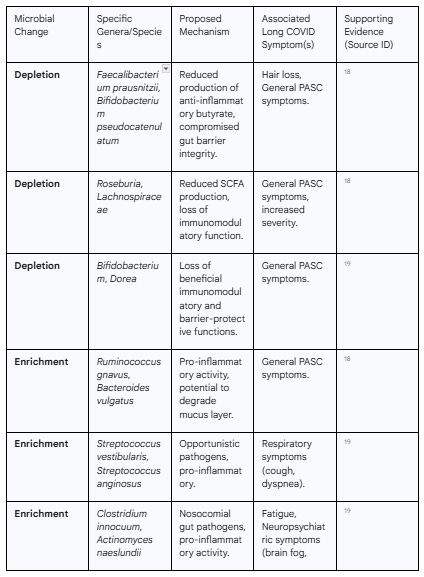
This signature is primarily characterized by a significant decrease in overall microbial diversity and a marked depletion of beneficial, symbiotic bacteria.18 Particularly affected are bacteria known for their ability to produce short-chain fatty acids (SCFAs), such as butyrate, which are crucial for maintaining gut health. Genera like Faecalibacterium, Bifidobacterium, and Roseburia are consistently found to be diminished in Long COVID patients.18 Butyrate is the primary energy source for colonocytes (the epithelial cells of the colon) and possesses potent anti-inflammatory properties; its depletion therefore weakens the gut barrier and promotes inflammation.20
Concurrently, this loss of beneficial microbes is accompanied by an enrichment of opportunistic pathogens and pro-inflammatory species. Bacteria such as Ruminococcus gnavus, Bacteroides vulgatus, and various species of Enterococcus and Streptococcus are often found in higher abundance in individuals with PASC.18 This dysbiotic state is not a transient feature of the acute illness; studies have shown that lower bacterial diversity and the pro-inflammatory microbial signature persist for at least 6 to 12 months in patients who develop Long COVID.18
Crucially, these specific microbial alterations have been directly correlated with specific Long COVID symptoms. For instance, an enrichment of opportunistic pathogens like Streptococcus has been linked to persistent respiratory symptoms, while depletion of butyrate-producing bacteria like F. prausnitzii has been associated with persistent hair loss.18 Neuropsychiatric symptoms and fatigue have been correlated with an increase in gut pathogens like Clostridium innocuum and Actinomyces naeslundii.19 This suggests that the specific nature of the gut dysbiosis may be a key determinant of an individual's Long COVID symptom cluster.
3.3. Compromised Intestinal Barrier Integrity and Microbial Translocation
The combination of persistent local inflammation and pro-inflammatory dysbiosis culminates in the physical breakdown of the intestinal epithelial barrier, a condition commonly referred to as increased intestinal permeability or "leaky gut".22 A key molecular driver of this process appears to be the protein zonulin, which acts as a physiological modulator of the tight junctions that seal the space between adjacent epithelial cells.22
Research in both pediatric Multisystem Inflammatory Syndrome in Children (MIS-C) and adult Long COVID has demonstrated that persistent SARS-CoV-2 in the gut is associated with a significant increase in the release of zonulin.22 Elevated zonulin levels lead to the disassembly of tight junction protein complexes, effectively loosening the seals between cells and increasing the permeability of the gut barrier.22
This compromised barrier becomes a gateway for substances from the gut lumen to translocate into the bloodstream—substances that are normally contained within the intestine. This includes not only viral antigens like the spike protein, which are shed from the gut reservoir 14, but also a host of microbial products. Key among these are lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria, and (1→3)-β-D-glucan, a component of fungal cell walls.20 The translocation of these highly inflammatory molecules from the gut into the systemic circulation is a critical turning point, transforming a localized gut pathology into a systemic disease.
3.4. The Systemic Inflammatory Response: A "Two-Hit" Model
The simultaneous translocation of both viral antigens and microbial products from the leaky gut creates what can be described as a "two-hit" stimulus for systemic inflammation. The body's immune system is now forced to contend with multiple, distinct pro-inflammatory signals circulating in the bloodstream.
This dual challenge triggers a sustained, low-grade systemic inflammatory response. The presence of microbial components like LPS in the blood leads to elevated levels of biomarkers such as soluble CD14 (sCD14), which is released by monocytes in response to LPS stimulation.9 The overall inflammatory state is characterized by increased circulating levels of pro-inflammatory cytokines, including Interleukin-1 beta (IL−1β), Interleukin-6 (IL-6), and Tumor Necrosis Factor alpha (TNF−α).7 This chronic systemic inflammation, continuously fueled by the leakage from the compromised gut, is considered a central pillar of Long COVID pathophysiology, providing the biological basis for the widespread, multi-organ symptoms experienced by patients.12
The relationship between the viral reservoir and gut dysbiosis is likely not a one-way street but rather a bidirectional, self-perpetuating vicious cycle that maintains the chronic disease state. The initial viral infection and its persistence directly cause inflammation that disrupts the gut environment, leading to dysbiosis.7 However, the resulting dysbiotic state—characterized by the loss of beneficial butyrate-producing bacteria—further degrades the gut barrier and impairs local immune regulation, as butyrate is essential for both colonocyte health and its anti-inflammatory properties.7 This weakened, pro-inflammatory environment created by the dysbiosis may, in turn, make it easier for the SARS-CoV-2 reservoir to persist by compromising local antiviral defenses. This creates a detrimental feedback loop: the virus causes dysbiosis, and the dysbiosis facilitates continued viral persistence. This suggests that therapeutic strategies may need to address both the virus and the microbiome simultaneously to break the cycle. It also implies that an individual's pre-infection microbiome health could be a key factor in determining their risk of developing a persistent gut reservoir and Long COVID.
4. The Gut-Brain Axis: Translating Gut Pathology into Systemic Long COVID Symptomatology
The gut-brain axis, a complex bidirectional communication network linking the enteric and central nervous systems, serves as the primary conduit through which the gut-centric pathology of a persistent SARS-CoV-2 reservoir is translated into the debilitating systemic, and particularly neurological, symptoms of Long COVID.1 This network utilizes neural (e.g., vagus nerve), endocrine, and immune pathways to allow the gut and brain to constantly influence one another.30 In Long COVID, this axis becomes a pathway for pathology, explaining the frequent co-occurrence of gastrointestinal, cognitive, and psychiatric symptoms.
4.1. Neuroinflammation and Cognitive Dysfunction: The Link to "Brain Fog"
One of the most pervasive and debilitating symptoms of Long COVID is a constellation of cognitive deficits collectively termed "brain fog," which includes impaired memory, reduced concentration, and slowed mental processing.24 The gut-brain axis model provides a robust mechanistic explanation for this phenomenon. The chronic systemic inflammation originating from the leaky gut is a primary driver of neuroinflammation.12
Circulating pro-inflammatory cytokines (like IL-6 and TNF-α) and translocated microbial products (like LPS) can cross the blood-brain barrier (BBB), particularly a BBB that may have been made more permeable by the initial viral infection itself.24 Once in the central nervous system, these molecules activate the brain's resident immune cells, the microglia.29 Chronic microglial activation leads to a state of persistent neuroinflammation, which disrupts normal neuronal function, impairs synaptic plasticity, and interferes with cognitive processing.24 This sustained neuroinflammatory state, fueled by the ongoing pathology in the gut, is the leading biological hypothesis for the cognitive dysfunction characteristic of Long COVID.24
4.2. Vagus Nerve Signaling and Autonomic Dysfunction
The vagus nerve forms a direct neural highway between the gut and the brain, providing rapid, bidirectional communication.29 In a healthy state, it transmits information about the gut environment to the brain, influencing mood and homeostasis. In Long COVID, this pathway can become a conduit for pathological signals. Inflammation in the gut wall can trigger aberrant signaling up the vagus nerve to the brainstem, contributing to the autonomic nervous system dysfunction (dysautonomia) observed in many patients.28
Symptoms of dysautonomia can include postural orthostatic tachycardia syndrome (POTS), dizziness, and gastrointestinal motility issues.1 Studies in mouse models engineered to mimic Long COVID have demonstrated a measurable reduction in vagus nerve activity, which was associated with cognitive deficits.30 This suggests that impaired vagal signaling, driven by gut inflammation, is a key mechanism linking the gut reservoir to both autonomic and cognitive symptoms.
4.3. Dysregulation of Serotonin and Other Neurotransmitters
The gut is a major endocrine organ, and the gut microbiome plays a critical role in producing or modulating neurotransmitters that influence brain function. Approximately 90% of the body's serotonin, a key neurotransmitter involved in mood, sleep, and cognition, is produced in the gut.1 Emerging research has identified a systemic reduction in serotonin levels as a key feature of Long COVID.30
This serotonin depletion is likely multifactorial, driven by several gut-centric mechanisms. First, viral persistence and inflammation may directly impair the function of enterochromaffin cells, the gut cells that produce serotonin. Second, SARS-CoV-2's occupation of the ACE2 receptor disrupts the function of the co-dependent B0AT1 transporter, which is responsible for absorbing tryptophan—the essential amino acid precursor for serotonin synthesis—from the gut.7 Reduced tryptophan absorption leads directly to reduced serotonin production. This serotonin deficiency has been causally linked to Long COVID symptoms; it impairs vagus nerve signaling and leads to memory deficits in animal models.30 Remarkably, restoring serotonin levels in these models with antidepressant medication reversed the cognitive deficits, providing strong evidence for this specific gut-brain pathway.30
The gut-brain axis thus provides a powerful, unifying framework that reframes Long COVID from a disparate collection of symptoms into a cohesive syndrome with a central pathological axis. A patient presenting with diarrhea, brain fog, and anxiety might traditionally be seen by multiple specialists for separate issues. The gut-brain axis model posits a single, upstream root cause: a perturbed gut. The viral reservoir and resulting dysbiosis directly cause the gastrointestinal symptoms. This same gut pathology then transmits aberrant signals via the vagus nerve and circulating molecules (cytokines, LPS, reduced serotonin) to the brain, where they manifest as neuroinflammation (brain fog) and neurotransmitter imbalance (anxiety). This has profound implications for clinical practice, suggesting that treating the gut—by eradicating the virus, restoring the barrier, or modulating the microbiome—could become a primary therapy for the neurological and psychiatric symptoms of Long COVID.
5. Contextualizing the Gut Reservoir: Competing and Interacting Pathophysiological Hypotheses
The gut reservoir theory provides a compelling explanation for many features of Long COVID, but it is crucial to understand it within the broader landscape of PASC research. Several other major hypotheses have been proposed to explain the pathophysiology of the condition, and these are not mutually exclusive. In fact, the gut reservoir hypothesis can be seen as a potential upstream driver that initiates or exacerbates these other pathological processes, offering a unifying framework that can account for the disease's complexity and heterogeneity.
5.1. Overview of Leading Long COVID Hypotheses
Beyond the gut reservoir, several other key mechanisms are believed to contribute to Long COVID.2 These include:
Immune Dysregulation and Autoimmunity: This hypothesis posits that the initial SARS-CoV-2 infection triggers a persistent, dysregulated immune response that fails to return to homeostasis.12 This can manifest as chronic T-cell and myeloid cell activation, skewed cytokine profiles favoring a pro-inflammatory state, and the development of autoantibodies that attack the body's own tissues through mechanisms like molecular mimicry or bystander activation.25
Endothelial Dysfunction and Microclots: There is substantial evidence for widespread damage to the endothelium (the lining of blood vessels) in Long COVID.27 This endotheliitis can lead to impaired blood flow, a pro-coagulant state, and the formation of persistent, fibrin-amyloid microclots that may occlude small vessels, leading to tissue hypoxia and contributing to symptoms like fatigue and brain fog.27
Latent Virus Reactivation: The immunological stress and dysregulation caused by SARS-CoV-2 infection may allow for the reactivation of other latent viruses that are harbored by most of the population, most notably Epstein-Barr virus (EBV).27 Reactivated EBV has been associated with the fatigue commonly seen in Long COVID.
Mitochondrial Dysfunction: This theory suggests that SARS-CoV-2 infection directly or indirectly impairs the function of mitochondria, the powerhouses of the cell. This leads to a deficit in cellular energy production (ATP), which could be a primary driver of the profound fatigue and post-exertional malaise experienced by many patients.27
5.2. Interplay and Convergence of Mechanisms
Rather than viewing these hypotheses as competing, it is more accurate to see them as deeply interconnected components of a complex pathological network. The persistent gut reservoir can act as a central, upstream driver that initiates and sustains these other downstream processes.
Gut Reservoir → Immune Dysregulation: The continuous shedding of viral antigens from the gut provides the chronic stimulus necessary to drive the observed immune dysregulation. This persistent antigenic challenge can lead to T-cell exhaustion, maintain pro-inflammatory cytokine production, and create an inflammatory environment conducive to breaking self-tolerance, potentially leading to the generation of autoantibodies.12
Gut Reservoir → Endothelial Dysfunction: The link here is direct and powerful. As established, a leaky gut allows for the translocation of microbial products like LPS into the bloodstream. LPS is a potent activator of endothelial cells and a known trigger for inflammation and coagulation.20 Therefore, the gut reservoir, by causing barrier dysfunction, can directly contribute to the systemic endotheliitis and pro-thrombotic state seen in Long COVID.
Gut Reservoir → Latent Virus Reactivation: The chronic immune activation and T-cell dysfunction fueled by the gut reservoir can compromise the immune surveillance that is required to keep latent viruses like EBV in check. This creates an immunological environment that is permissive for their reactivation.27
This integrated perspective suggests that the remarkable heterogeneity of Long COVID, which can manifest with over 200 different symptoms, may be explained by the differential dominance of these interconnected pathways in different individuals. While the gut reservoir may be a common initiating event, an individual's genetic background, pre-existing comorbidities, and microbiome composition could determine which downstream pathological cascade predominates. For one patient, the primary consequence might be massive LPS translocation leading to a clinical picture dominated by endothelial dysfunction and microclots. For another, the persistent antigen may primarily trigger an autoimmune response, resulting in a rheumatological-like presentation. For a third, severe dysbiosis and serotonin disruption could lead to a predominantly neuropsychiatric phenotype. This has critical implications for clinical research, highlighting the need for careful patient stratification in therapeutic trials. Lumping all Long COVID patients together without regard for their underlying pathophysiology may cause promising treatments to appear ineffective.
6. Therapeutic Strategies Targeting the Gut Reservoir and its Sequelae
The gut-centric model of Long COVID pathophysiology provides a clear and actionable framework for developing therapeutic interventions. Strategies can be aimed at different points in the pathological cascade: directly eradicating the viral reservoir, restoring the integrity of the gut barrier, modulating the dysbiotic microbiome, and mitigating the downstream systemic inflammation.
6.1. Eradicating the Reservoir: The Rationale for Antiviral Therapies
The most direct therapeutic approach is to eliminate the source of the chronic antigenic stimulation: the persistent SARS-CoV-2 reservoir itself. This provides a strong rationale for testing antiviral medications in patients with Long COVID.15 Several clinical trials are underway to investigate this hypothesis. The large-scale RECOVER initiative includes a trial platform specifically designed to test interventions for viral persistence.33 Smaller, investigator-initiated trials are also exploring this avenue, such as the PREVAIL-LC trial, a placebo-controlled study of the antiviral ensitrelvir in Long COVID patients.34 Early data on the use of nirmatrelvir-ritonavir (Paxlovid) during the acute phase of infection suggests it may reduce the risk of developing PASC, lending further support to the idea that controlling viral replication is key.35 If these trials demonstrate that clearing the reservoir leads to symptom resolution, it would provide definitive evidence for the viral persistence hypothesis.
6.2. Restoring the Barrier: Novel Approaches to Enhance Gut Integrity
If the translocation of viral and microbial products is a key driver of systemic inflammation, then therapies aimed at restoring the integrity of the gut barrier are a logical intervention. Research has identified the zonulin pathway as a critical regulator of intestinal tight junctions and a potential therapeutic target.22 Larazotide is a zonulin antagonist that works by preventing the breakdown of tight junctions. In a proof-of-concept study involving children with MIS-C—a condition with parallels to Long COVID—treatment with larazotide led to more rapid clearance of SARS-CoV-2 spike protein from the blood and faster improvement of gastrointestinal symptoms compared to untreated controls.22 This suggests that "sealing the leak" can prevent the trafficking of inflammatory triggers from the gut into the bloodstream and represents a promising therapeutic strategy for Long COVID.
6.3. Modulating the Microbiome: Probiotics, Prebiotics, and Fecal Microbiota Transplantation (FMT)
Given the profound and persistent dysbiosis associated with Long COVID, interventions designed to restore a healthy gut microbiome are being actively investigated.21 These strategies include the use of probiotics, prebiotics, and synbiotics (combinations of both), as well as fecal microbiota transplantation (FMT).
A large, randomized, double-blind, placebo-controlled trial of a specific synbiotic formulation (SIM01) in Long COVID patients demonstrated significant efficacy. Over a six-month period, the group receiving the synbiotic showed significantly greater improvement in fatigue, memory loss, difficulty concentrating, and gastrointestinal upset compared to the placebo group.21 The treatment was shown to increase gut microbial diversity and enrich for beneficial, butyrate-producing bacteria like Bifidobacterium and Faecalibacterium prausnitzii.21 Other studies have shown benefits from different probiotic combinations for post-COVID fatigue.21
FMT, a more wholesale approach to microbiome restoration, has also shown promise in smaller studies. A prospective interventional study found that FMT was effective in alleviating post-COVID insomnia and anxiety.21 These findings collectively suggest that correcting the dysbiosis is a viable therapeutic strategy that can ameliorate a wide range of Long COVID symptoms, likely by reducing local gut inflammation, enhancing barrier function, and favorably modulating gut-brain axis signaling.21
6.4. Targeting Downstream Inflammation
Finally, even if the upstream drivers cannot be immediately eliminated, it may be possible to target the downstream inflammatory consequences. For example, research has shown that the translocation of fungal products like (1→3)-β-D-glucan from the gut contributes to systemic inflammation in Long COVID.23 The inflammatory signaling pathways activated by β-glucan are well-characterized and can potentially be blocked by small molecule inhibitors. This approach would not address the root cause but could help manage the chronic inflammatory state and its associated symptoms while other therapies targeting the reservoir or the barrier take effect.23
7. Conclusion and Future Directions
7.1. Synthesizing the Evidence: A Unified Model of Gut-Driven Pathophysiology in Long COVID
The cumulative evidence strongly supports a model in which a persistent SARS-CoV-2 reservoir in the gastrointestinal tract serves as a central driver of pathophysiology in a significant proportion of Long COVID patients. This model provides a coherent narrative that connects the virus's initial tropism for the ACE2-rich gut to the multi-systemic and often debilitating chronic symptoms of the disease. The establishment of this reservoir initiates a self-sustaining vicious cycle of localized inflammation, profound gut dysbiosis, and a breach of the intestinal barrier. The resulting translocation of viral and microbial products into the systemic circulation fuels a chronic, low-grade inflammatory state. This systemic inflammation, propagated and translated into specific clinical manifestations through pathways like the gut-brain axis, can account for the hallmark symptoms of Long COVID, from gastrointestinal distress to profound fatigue and cognitive "brain fog." This gut-centric framework does not exclude other pathophysiological mechanisms but rather provides a unifying upstream driver that can initiate and sustain processes such as broader immune dysregulation, endothelial dysfunction, and autonomic nervous system impairment.
7.2. Identifying Knowledge Gaps and Priorities for Future Research
Despite the compelling progress, critical knowledge gaps remain, and addressing them should be a priority for the research community.
Standardized Biomarkers: There is an urgent need to develop and validate standardized, non-invasive biomarkers that can reliably identify the presence of a persistent viral reservoir.32 Ultrasensitive assays for circulating viral proteins (like spike) or specific patterns of tissue-resident T-cell activation in the blood could help stratify patients for clinical trials and guide personalized treatment.37
Advanced Animal Models: A major limitation in the field is the lack of robust small animal models that faithfully replicate the gut persistence and chronic symptomatology of Long COVID.13 Developing such models is essential for dissecting the precise molecular mechanisms of persistence and for the preclinical testing of novel therapeutics.13
Understanding Host Predisposition: It is crucial to understand the host factors—genetic, immunological, and microbiome-related—that determine why some individuals successfully clear the virus from the gut while others develop a persistent reservoir. Large-scale longitudinal studies that characterize patients before, during, and after infection are needed to identify these predisposing factors.
Well-Stratified Clinical Trials: Future therapeutic trials must move beyond a one-size-fits-all approach. It is imperative that trials for antivirals, barrier-repair agents, or microbiome modulators enroll patients who are stratified based on biomarkers indicating the presence of the specific pathology being targeted (e.g., evidence of viral persistence for an antiviral trial).32
Ultimately, the research agenda must be focused on moving from correlation to establishing definitive causation. By elucidating the precise mechanisms linking the gut reservoir to systemic disease and by validating therapeutic strategies in rigorously designed clinical trials, it will be possible to translate these crucial pathophysiological insights into effective treatments for the millions of individuals affected by Long COVID.
Acknowledgement
I acknowledge the use of Gemini AI in the preparation of this report. Specifically, it was used to: (1) brainstorm and refine the initial research questions; (2) assist in writing and debugging Python scripts for statistical analysis; and (3) help draft, paraphrase, and proofread sections of the final manuscript. I reviewed, edited, and assume full responsibility for all content.
Works cited
The Intestine in Acute and Long COVID: Pathophysiological Insights ..., accessed September 9, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11650913/
Mechanisms of Gut-Related Viral Persistence in Long COVID - PMC - PubMed Central, accessed September 9, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11360392/
Intestinal Study Yields Insights Into Persistence of SARS-CoV-2, accessed September 9, 2025, https://reports.mountsinai.org/article/gi2022-_6_evolution-of-antibody-immunity-in-the-bowel
Role of Gut Microbiota in Long COVID: Impact on Immune Function and Organ System Health - PubMed Central, accessed September 9, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11883900/
Direct Evidence of Active SARS-CoV-2 Replication in the Intestine ..., accessed September 9, 2025, https://academic.oup.com/cid/article/73/3/361/5868547
Gut as viral reservoir: lessons from gut viromes, HIV and COVID-19 ..., accessed September 9, 2025, https://gut.bmj.com/content/70/9/1605
Long-Term Effects of COVID-19 on the Gastrointestinal System ..., accessed September 9, 2025, https://www.ptglab.com/news/blog/long-term-effects-of-covid-19-on-the-gastrointestinal-system/
Gut microbiome and clinical and lifestyle host factors associated with recurrent positive RT-PCR for SARS-CoV-2 - Frontiers, accessed September 9, 2025, https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2024.1494193/full
SARS-CoV-2 infection perturbs the gastrointestinal tract and induces modest microbial translocation across the intestinal barrier - ASM Journals, accessed September 9, 2025, https://journals.asm.org/doi/10.1128/jvi.01288-24
Investigating the role of SARS-CoV-2 persistence in the ..., accessed September 9, 2025, https://polybio.org/projects/investigating-the-role-of-sars-cov-2-persistence-in-the-gastrointestinal-tract-in-the-pathogenesis-of-long-covid/
(PDF) Mechanisms of Gut-Related Viral Persistence in Long COVID - ResearchGate, accessed September 9, 2025, https://www.researchgate.net/publication/382986786_Mechanisms_of_Gut-Related_Viral_Persistence_in_Long_COVID
Long COVID: A proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome | Request PDF - ResearchGate, accessed September 9, 2025, https://www.researchgate.net/publication/360448050_Long_COVID_A_proposed_hypothesis-driven_model_of_viral_persistence_for_the_pathophysiology_of_the_syndrome
Long COVID: Defining the viral RNA reservoir in the gastrointestinal tract, accessed September 9, 2025, https://polybio.org/projects/long-covid-defining-the-viral-rna-reservoir-in-the-gastrointestinal-tract/
Are pockets of Covid in the gut causing long-term symptoms? | Long ..., accessed September 9, 2025, https://www.theguardian.com/society/2022/jun/28/are-pockets-of-covid-in-the-gut-causing-long-term-symptoms
Scientists detail current evidence that Long COVID is caused by persistent SARS-CoV-2 viral reservoirs - BioSpace, accessed September 9, 2025, https://www.biospace.com/scientists-detail-current-evidence-that-long-covid-is-caused-by-persistent-sars-cov-2-viral-reservoirs
Gut as an Alternative Entry Route for SARS-CoV-2: Current Evidence and Uncertainties of Productive Enteric Infection in COVID-19 - MDPI, accessed September 9, 2025, https://www.mdpi.com/2077-0383/11/19/5691
Impact of SARS-CoV-2 on Long COVID gut and lymphoid pathology ..., accessed September 9, 2025, https://polybio.org/projects/impact-of-sars-cov-2-on-long-covid-gut-and-lymphoid-pathology/
Gut Microbiome Disruption Following SARS-CoV-2: A Review - MDPI, accessed September 9, 2025, https://www.mdpi.com/2076-2607/12/1/131
Full article: Long COVID and gut microbiome: insights into pathogenesis and therapeutics, accessed September 9, 2025, https://www.tandfonline.com/doi/full/10.1080/19490976.2025.2457495
SARS-CoV-2 infection is associated with intestinal permeability, systemic inflammation, and microbial dysbiosis in hospitalized patients | Microbiology Spectrum - ASM Journals, accessed September 9, 2025, https://journals.asm.org/doi/10.1128/spectrum.00680-24
(PDF) Long COVID and gut microbiome: insights into pathogenesis ..., accessed September 9, 2025, https://www.researchgate.net/publication/388362251_Long_COVID_and_gut_microbiome_insights_into_pathogenesis_and_therapeutics
SARS-CoV-2 gut persistence, intestinal permeability, and spike protein in pediatric Long COVID & MIS-C - PolyBio Research Foundation, accessed September 9, 2025, https://polybio.org/projects/sars-cov-2-gut-persistence-intestinal-permeability-and-spike-protein-in-pediatric-long-covid-mis-c/
Looking Inside the Gut for Answers to Long-COVID - The Wistar Institute, accessed September 9, 2025, https://www.wistar.org/featured-news/looking-inside-the-gut-for-answers-to-long-covid/
Gut-brain pathogenesis of post-acute COVID-19 ... - Frontiers, accessed September 9, 2025, https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2023.1232480/full
A review of cytokine-based pathophysiology of Long COVID symptoms - Frontiers, accessed September 9, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1011936/full
Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement - MDPI, accessed September 9, 2025, https://www.mdpi.com/1422-0067/25/12/6389
Mechanisms of long COVID: An updated review - PMC, accessed September 9, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11332859/
Long COVID: Clinical characteristics, proposed ... - Frontiers, accessed September 9, 2025, https://www.frontiersin.org/journals/molecular-biosciences/articles/10.3389/fmolb.2023.1157651/full
A Comprehensive Narrative Review on Long COVID-19 Syndrome ..., accessed September 9, 2025, https://directivepublications.org/taoim/articles/A-Comprehensive-Narrative-Review-on-Long-COVID-19-Syndrome-and-the-Gut-Brain-Axis-A-New-Frontier-in-Post-Viral-Syndromes.pdf
The gut-brain connection: What the science says - Stanford Medicine, accessed September 9, 2025, https://med.stanford.edu/news/insights/2025/03/gut-brain-connection-long-covid-anxiety-parkinsons.html
Role of the microbiota-gut-brain axis in postacute COVID syndrome - PMC - PubMed Central, accessed September 9, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10042594/
Targeting the SARS-CoV-2 reservoir in long COVID. | J. Craig Venter Institute, accessed September 9, 2025, https://www.jcvi.org/publications/targeting-sars-cov-2-reservoir-long-covid
RECOVER Clinical Trials | Home, accessed September 9, 2025,
https://trials.recovercovid.org/
UCSF COVID-19 Trial → Ensitrelvir for Viral Persistence and ..., accessed September 9, 2025, https://clinicaltrials.ucsf.edu/trial/NCT06161688
Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative - PMC, accessed September 9, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10034329/
Development and management of gastrointestinal symptoms in long-term COVID-19, accessed September 9, 2025, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2023.1278479/full
New $2.1 million grant to fund long COVID research - Penn Medicine, accessed September 9, 2025, https://www.pennmedicine.org/news/new-grant-to-fund-long-covid-research-on-gut-and-immune-response