Precision Immune Re-calibration: A New Therapeutic Paradigm Beyond Immunosuppression
Immune therapies are shifting from broad suppression to precise re-calibration—targeting specific cells, pathways, and restoring balance for better outcomes with fewer side effects.
The Principles of Immune Homeostasis and Its Pathological Disruption
The intricate network of the immune system is tasked with a dual mandate of paramount importance: to mount a robust defense against an endless array of foreign pathogens while simultaneously maintaining a state of non-reactivity, or tolerance, to the body's own tissues. The successful execution of this mandate results in a state of immune homeostasis, a concept central to health and disease. This is not a passive or static condition but rather a dynamic and actively maintained equilibrium, orchestrated by a complex interplay of cellular and molecular regulators. A failure to maintain this delicate balance leads to a spectrum of pathologies, ranging from autoimmune diseases, where the system over-reacts against self, to cancer and chronic infections, where it under-reacts to legitimate threats. For decades, the primary therapeutic strategy for immune-mediated diseases has been broad immunosuppression, a blunt approach that, while effective at dampening inflammation, carries a significant burden of toxicity and fails to address the root cause of the imbalance. The modern era of immunology is defined by a paradigm shift away from this approach towards precision immune re-calibration—a sophisticated strategy that seeks not to dismantle the immune system, but to correct its specific dysfunctions and restore its natural homeostatic balance.
Defining Immune Homeostasis: A Dynamic Equilibrium of Activation, Tolerance, and Regulation
Immune homeostasis is best understood as a state of equilibrium, or a tendency to reach equilibrium, maintained by a network of innate and adaptive immune cells that continually monitor their environment.1 This system is perpetually engaged in distinguishing "self" from "non-self," establishing intricate cell-to-cell communication networks to protect the organism from pathogens, toxins, and neoplastic cells.1 The balance is maintained through a variety of tightly regulated mechanisms that ensure immune responses are both effective and appropriate, preventing the excessive or inappropriate reactions that can lead to tissue damage.2
Central to this regulatory architecture are specialized cell types and molecular pathways. Regulatory T-cells (Tregs), a distinct subset of CD4$^{+}$ T lymphocytes, are essential guardians of self-tolerance. They actively suppress overactive immune responses, inhibiting the activation of other immune cells that could potentially cause autoimmunity or chronic inflammation.2 Their function is complemented by a series of molecular "brakes" known as immune checkpoints. Receptors like Programmed cell death protein 1 (PD-1), when engaged by their ligands, deliver inhibitory signals that terminate T-cell activation, preventing runaway immune responses.5 The production of anti-inflammatory cytokines, such as Interleukin-10 (IL-10) and Transforming growth factor-beta (TGF-β), further contributes to this regulatory milieu, directly inhibiting the function of pro-inflammatory cells and promoting a state of tolerance.7
This homeostatic balance is not an isolated, intrinsic property of the immune system but is profoundly influenced by systemic factors. The gut microbiota, for instance, has co-evolved with the host immune system and plays a crucial role in its education and calibration.9 Beneficial bacteria promote tolerance and regulate inflammation, in part by producing metabolites like short-chain fatty acids (SCFAs) that can stimulate the activity of Tregs.2 This constant dialogue between the host and its microbial commensals underscores that immune homeostasis is a negotiated state, continuously adapting to both internal and external environmental cues. The system is not designed to be inert but to buffer responses against extremes, maintaining a level of cross-reactivity that allows for rapid reactions to a broad range of antigens while preventing self-destruction.1 This active, energy-dependent process of regulation is fundamental to health; its failure is not merely an "attack" but a breakdown of these complex control networks, necessitating therapeutic interventions that can restore, rather than simply suppress, their function.
The Consequences of Dysregulation: A Spectrum of Disease
When the intricate mechanisms governing immune homeostasis are disrupted, the system can shift towards a new, pathological state of equilibrium, resulting in disease.1 This dysregulation can manifest as either an over-reactive or an under-reactive immune response.
Immune Over-Reactivity (Autoimmunity and Allergy): In conditions of over-reactivity, the principle of self-tolerance fails. The immune system mistakenly identifies healthy host cells and tissues as foreign, mounting a sustained attack that leads to chronic inflammation and tissue damage. This is the hallmark of autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), and psoriasis.1 The triggers for this breakdown in tolerance are multifactorial and can include a combination of genetic susceptibility, stochastic environmental events, and molecular mimicry, where an immune response to a pathogen cross-reacts with a structurally similar self-antigen.1 Similarly, allergic conditions like asthma and eczema represent an over-reaction to otherwise harmless environmental antigens.12
Immune Under-Reactivity (Cancer and Chronic Infection): At the opposite end of the spectrum, an inefficient, unresponsive, or exhausted immune system fails in its surveillance role. This hypo-reactivity can allow aberrant, malignant cells to evade detection and elimination, resulting in the uncontrolled growth of tumors.1 A similar failure can occur in the context of persistent pathogens. In chronic infections, such as those caused by Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), or Hepatitis C Virus (HCV), the continuous presence of antigens can drive T-cells into a state of dysfunction known as T-cell exhaustion, limiting the immune-mediated clearance of the pathogen.1 In both cancer and chronic infection, the immune system is unable to mount a sufficiently potent and sustained response to restore homeostasis.
In many of these disease states, a common pathological feature is unresolved inflammation. The uncontrolled release of pro-inflammatory cytokines can create a vicious cycle, perpetuating tissue damage and further dysregulating the immune response, whether the initial problem was one of over- or under-reactivity.15
The Limitations of Broad Immunosuppression: A Paradigm in Transition
For many years, the standard of care for autoimmune and inflammatory disorders has relied on traditional immunosuppressants. This category includes conventional disease-modifying antirheumatic drugs (DMARDs) like methotrexate and azathioprine, as well as corticosteroids like dexamethasone.12 These agents function by exerting a broad, non-selective dampening effect on the immune system.17 Methotrexate, for example, interferes with the rapid growth of immune cells, while corticosteroids have widespread immunosuppressive and anti-inflammatory activities.12
While these drugs can be effective in reducing the symptoms of inflammation, their lack of specificity is their greatest liability. By weakening the entire immune system, they compromise the body's ability to fight off legitimate threats, leaving patients highly vulnerable to viral, bacterial, and fungal infections.12 This necessitates stringent precautions, such as avoiding sick individuals and maintaining rigorous hygiene, to mitigate the risk of life-threatening complications.12 Furthermore, their broad mechanism of action leads to a host of off-target side effects, ranging from common issues like fatigue, nausea, and headaches to more severe organ toxicity.12
Perhaps most importantly, these therapies often manage symptoms without correcting the underlying immunological defect. They may slow disease progression but rarely induce deep, durable, drug-free remission.18 This has driven a critical evolution in therapeutic strategy, reflected in the very language used to describe these medicines. The distinction between a traditional "immunosuppressant" and a modern "immunomodulator" is more than semantic; it represents a fundamental shift in philosophy. While regulatory classifications can be inconsistent, with different agencies labeling the same drug differently, the conceptual difference is clear.17 The old paradigm used a "broad brush" to suppress the entire system.17 The new paradigm, which encompasses biologics and other targeted therapies, aims for "precise intervention" by targeting the specific cells, signaling pathways, or inflammatory mediators implicated in the disease process.3 This pursuit of specificity promises not only to enhance therapeutic efficacy but also to minimize the debilitating side effects of global immunosuppression, moving the field toward the ultimate goal of "precise calibration" to restore true immune homeostasis.3
Reinvigorating T-Cell Function by Targeting Exhaustion Pathways
In the landscape of chronic diseases, particularly cancer and persistent viral infections, the failure of the immune system is often not due to an absence of T-cells, but to their functional incapacitation. This state, known as T-cell exhaustion, represents a critical barrier to effective immunity. It is a complex process of cellular reprogramming that renders T-cells unable to perform their effector functions. However, pioneering research has revealed that this exhausted state is not always irreversible. By targeting the specific molecular pathways that induce and maintain exhaustion, it is possible to "reinvigorate" these dysfunctional T-cells, unleashing their potent therapeutic potential. This strategy, exemplified by the success of immune checkpoint inhibitors, forms a cornerstone of modern immunotherapy, yet it also exposes the delicate balance between activating anti-disease immunity and triggering autoimmunity.
The Molecular Landscape of T-Cell Exhaustion (Tex)
T-cell exhaustion is a state of T-cell dysfunction that arises as an adaptive response to persistent antigen stimulation and chronic inflammation.23 Initially observed in the context of chronic viral infections, it is now recognized as a central mechanism of immune evasion by tumors.24 Rather than being a simple state of cellular fatigue, exhaustion is an alternative T-cell differentiation pathway, resulting in a cell population with a distinct and stable epigenetic, transcriptional, and metabolic profile.26 Exhausted T-cells (Tex) are characterized by a hierarchical and progressive loss of effector functions. The capacity to produce IL-2 and proliferate is lost early, followed by a decline in the production of TNF-α and, finally, IFN-γ.23 This functional decline is accompanied by poor responsiveness to homeostatic cytokines like IL-7 and IL-15, which are crucial for maintaining memory T-cell populations.23 Challenging the long-held belief that exhaustion is a protracted process developing over weeks or months, recent evidence from mouse models suggests that the key hallmarks of T-cell dysfunction can be imprinted within hours of initial exposure to a tumor, indicating a rapid and decisive programming event.27
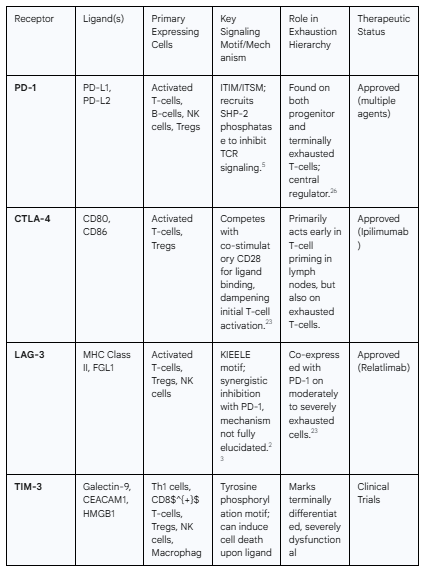
The most prominent molecular hallmark of T-cell exhaustion is the sustained, high-level co-expression of multiple inhibitory receptors, which act as molecular "brakes" on T-cell activation.23 These receptors, when engaged by their respective ligands in the tissue microenvironment, deliver potent inhibitory signals that override T-cell receptor (TCR) activation. The key inhibitory receptors include:
PD-1 (Programmed cell death protein 1; PDCD1): The canonical marker of exhaustion. Its interaction with its ligands, PD-L1 and PD-L2, is a primary driver of T-cell dysfunction. PD-1 signaling inhibits TCR-mediated activation signals and suppresses the production of effector cytokines.5
LAG-3 (Lymphocyte-activation gene 3): Frequently co-expressed with PD-1 on exhausted T-cells, LAG-3 binds to MHC class II molecules and delivers an independent inhibitory signal, contributing to T-cell hypofunction.23
TIM-3 (T-cell immunoglobulin and mucin-domain containing-3; HAVCR2): Often marks a more terminally differentiated and severely dysfunctional state of exhaustion. Its ligand, galectin-9, can induce T-cell apoptosis.23
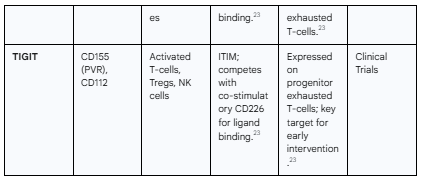
TIGIT (T cell immunoreceptor with Ig and ITIM domains): An emerging and critical checkpoint that competes with the co-stimulatory receptor CD226 for binding to their shared ligand, PVR (CD155). TIGIT expression marks exhausted T-cells and directly inhibits their function.23
Other markers: A host of other receptors, including CTLA-4, 2B4 (CD244), CD160, and LAYN, as well as the chemokine CXCL13, are also associated with the exhausted phenotype, forming a complex signature that defines this dysfunctional state.23
This exhausted state is not a bug in the system, but rather a feature. It is a physiological regulatory mechanism designed to limit immunopathology and tissue damage during situations of chronic inflammation, such as in the placenta or in the context of autoimmunity.24 Tumors and chronic viruses do not simply wear T-cells out; they exploit and hijack this pre-existing, adaptive "off-switch" to ensure their own survival. This understanding is crucial, as it reframes the therapeutic challenge: the goal is not merely to "boost" T-cell activity, but to selectively disable a fundamental safety mechanism of the immune system in a context-dependent manner.
Therapeutic Reversal via Immune Checkpoint Blockade (ICB)
The discovery that T-cell exhaustion is an actively maintained state, rather than a terminal one, opened the door to a revolutionary therapeutic strategy: immune checkpoint blockade (ICB). The principle of ICB is to use monoclonal antibodies to physically block the interaction between an inhibitory receptor on the T-cell (e.g., PD-1) and its corresponding ligand (e.g., PD-L1) on a tumor cell or antigen-presenting cell.14 This blockade effectively "releases the brakes" on the T-cell, preventing the delivery of the inhibitory signal and restoring its capacity to proliferate, produce effector cytokines, and execute its cytotoxic functions.25
This approach has transformed the field of oncology. Immune checkpoint inhibitors targeting the PD-1/PD-L1 and CTLA-4 pathways have demonstrated unprecedented and durable responses in a wide range of malignancies, including melanoma, non-small cell lung cancer, and renal cell carcinoma, leading to a paradigm shift in cancer treatment.14 The success of ICB is predicated on its ability to "un-exhaust" or "revive" the patient's own pre-existing, tumor-specific T-cells that had been rendered dysfunctional within the tumor microenvironment.25
The same logic applies to chronic infectious diseases, where T-cell exhaustion is a key mechanism of pathogen persistence. Preclinical and early clinical studies have shown that blocking the PD-1 pathway can enhance T-cell function and improve control of various pathogens. In models of chronic viral infection (HBV, HCV, HIV), bacterial infection (H. pylori), and parasitic infection (Toxoplasma gondii, Leishmania), PD-1 blockade has been shown to increase T-cell proliferation and cytokine production, leading to a reduction in pathogen burden.6 This suggests that ICB could become a valuable therapeutic modality for infectious diseases that are difficult to clear with conventional antimicrobial agents.
The Double-Edged Sword: PD-1's Role in Autoimmunity and the Challenge of irAEs
The remarkable success of immune checkpoint inhibitors comes with a significant and mechanistically informative caveat: immune-related adverse events (irAEs). The very biological pathway that ICIs disrupt to unleash anti-tumor immunity is the same pathway that the body uses to maintain self-tolerance and prevent autoimmunity.5 The physiological role of PD-1 is to act as a guardian of peripheral tolerance, suppressing the activation of self-reactive T-cells that escape central tolerance mechanisms in the thymus.6 This is starkly demonstrated in preclinical models, where mice genetically deficient in PD-1 spontaneously develop a spectrum of autoimmune-like diseases, including lupus-like arthritis and glomerulonephritis.5
Therefore, when a patient is treated with a PD-1 inhibitor, the therapeutic effect and the primary toxicity are two sides of the same coin. The blockade of PD-1 reinvigorates not only the anti-tumor T-cells but also any latent autoreactive T-cells, leading to iatrogenic autoimmune disease. These irAEs can affect virtually any organ system, commonly manifesting as colitis, hepatitis, pneumonitis, dermatitis, and a range of endocrinopathies, including autoimmune thyroiditis and fulminant type 1 diabetes.19 The mechanisms driving irAEs are thought to mirror those of spontaneous autoimmunity, involving the activation of cytotoxic T-cells against antigens shared between tumors and normal tissues, a disruption of Treg homeostasis, and the activation of autoantibody-producing B-cells.19
This inextricable link between efficacy and toxicity highlights the central challenge for the future of immunotherapy. Patients with pre-existing autoimmune diseases, who are often excluded from pivotal clinical trials, are at a particularly high risk of severe disease flares or new irAEs when treated with ICIs.19 The future of this therapeutic class will depend on developing strategies that can decouple the desired anti-tumor effect from systemic autoimmune toxicity. This may involve tumor-targeted delivery of checkpoint inhibitors, the development of bispecific antibodies that are only active within the tumor microenvironment, or combination therapies that can locally enhance immunity while systemically maintaining tolerance.
Precision Targeting of B-Lymphocytes in Autoantibody-Mediated Disease
While T-cells are often the primary drivers of cellular autoimmunity, B-lymphocytes play an equally critical, multifaceted role in the pathogenesis of many autoimmune diseases. Their contributions extend far beyond their well-established function as precursors to antibody-secreting plasma cells. B-cells are also potent antigen-presenting cells (APCs) that activate autoreactive T-cells and can secrete pro-inflammatory cytokines, creating a feedback loop that perpetuates chronic inflammation. Consequently, targeting B-cells has become a cornerstone of therapy for diseases like rheumatoid arthritis and lupus. However, initial strategies based on the broad depletion of B-cell populations have revealed significant limitations, primarily stemming from a lack of specificity. The field is now rapidly advancing toward highly precise, next-generation therapies designed to selectively eliminate only the pathogenic, autoantibody-producing B-cells, or even to restore B-cell tolerance, heralding a new era of precision medicine for these debilitating conditions.
Beyond Pan-Depletion: The Limitations of Anti-CD20 and Anti-BAFF Therapies
The first generation of targeted B-cell therapies focused on depleting or inhibiting large swathes of the B-cell compartment. Rituximab, a monoclonal antibody targeting the CD20 molecule expressed on the surface of most B-cells, and belimumab, which neutralizes B-cell Activating Factor (BAFF), a critical survival cytokine for B-cells, have demonstrated clinical efficacy in several autoimmune disorders.34 These therapies work by inducing a widespread reduction in B-cell numbers, thereby diminishing their capacity to produce autoantibodies, present antigens, and secrete cytokines.34
Despite their successes, these pan-B-cell depletion strategies are fundamentally flawed by their imprecision. Their key limitations include:
Non-Specificity: By targeting markers like CD20, these drugs eliminate both the harmful, autoreactive B-cells and the beneficial, protective B-cells required for normal immune defense. This global suppression of humoral immunity impairs the ability to mount effective responses to new pathogens and vaccines, increasing the risk of infection.34
Sparing of Pathogenic Plasma Cells: A critical biological limitation is that terminally differentiated, long-lived plasma cells—the primary factories for autoantibody production—downregulate CD20 expression. Consequently, anti-CD20 therapies like rituximab fail to eliminate these key pathogenic cells.34 This directly explains why these treatments often result in incomplete reduction of autoantibody titers and why patients frequently relapse as new B-cells differentiate from the untouched plasma cell reservoir.34 The failure of these therapies is therefore not one of potency, but of precision, as they miss the most critical cellular culprits.
Incomplete Tissue Depletion: Evidence suggests that anti-CD20 antibodies may not efficiently penetrate and eliminate B-cells residing in certain tissue compartments, such as the salivary glands in Sjögren's syndrome, allowing pathogenic cells to persist in protected niches.36
These limitations have been the primary impetus for developing more sophisticated approaches that can distinguish friend from foe within the B-cell population.
Antigen-Specific Depletion Strategies: The Next Generation
The central goal of next-generation B-cell therapies is to achieve exquisite specificity: to selectively deplete or silence only the B-cell clones whose B-cell receptors (BCRs) recognize the specific autoantigen driving the disease, while leaving the vast remainder of the protective B-cell repertoire intact.34 This is achieved through a paradigm-inverting strategy: instead of using a therapeutic antibody to target a generic cell surface marker, these approaches use the autoantigen itself as a high-precision "homing device" to deliver a therapeutic payload exclusively to the cells that recognize it.
Chimeric Autoantibody Receptor (CAAR) T-Cells: This groundbreaking cellular therapy represents the pinnacle of this antigen-specific approach. It is a direct adaptation of the Chimeric Antigen Receptor (CAR) T-cell technology that has revolutionized oncology. In CAAR-T therapy, a patient's own T-cells are genetically engineered to express a CAAR. The innovation lies in the design of the receptor's extracellular domain: instead of an antibody fragment that recognizes a tumor antigen, it consists of the autoantigen itself.21 For example, in the autoimmune blistering disease pemphigus vulgaris, which is caused by antibodies against the skin protein desmoglein-3 (Dsg3), the CAAR incorporates the Dsg3 protein. These engineered CAAR-T cells can then recognize, bind to, and kill only the B-cells that are displaying anti-Dsg3 antibodies on their surface (i.e., the pathogenic B-cells). This approach has demonstrated remarkable specificity in preclinical models and is now being evaluated in early-phase clinical trials for pemphigus vulgaris and myasthenia gravis, offering the potential for a highly targeted and durable remission.21
Engineered Biologics and Nanoparticles: Beyond cellular therapies, similar principles of antigen-specific targeting are being applied to develop novel biologics and drug delivery systems.
Antigen-Drug Conjugates: Analogous to antibody-drug conjugates (ADCs) used in cancer, this strategy involves chemically linking the autoantigen to a potent cytotoxic drug. When the autoreactive B-cell binds the antigen via its BCR, it internalizes the entire complex, delivering the toxic payload directly into the cell and triggering its death.34
Tolerogenic Nanoparticles (tNPs): Synthetic nanoparticles can be engineered to function as antigen-specific inhibitory platforms. The surface of the nanoparticle is decorated with the autoantigen to ensure binding to the correct B-cell. The nanoparticle can also carry an inhibitory cargo, such as the drug rapamycin or a ligand for an inhibitory B-cell co-receptor like CD22. Upon binding, the tNP delivers a potent signal that induces a state of unresponsiveness (anergy) or programmed cell death (apoptosis) specifically in the autoreactive B-cell.34
Bispecific Antibodies: Innovative antibody engineering has produced bispecific molecules designed to deliver an inhibitory signal to autoreactive B-cells. For instance, the investigational drug PRV-3279 is designed to simultaneously bind to a component of the BCR complex (CD79b) and an inhibitory receptor (Fc$\gamma$RIIb) on the B-cell surface. This co-engagement cross-links the two receptors, delivering a powerful "off" signal that prevents B-cell activation without causing cell death.34
Restoring B-Cell Tolerance: Re-educating the Immune System
An even more sophisticated approach moves beyond simply killing pathogenic B-cells to actively restoring the natural tolerance mechanisms that have failed. The development of B-cells is governed by a series of quality control checkpoints in the bone marrow (central tolerance) and peripheral lymphoid organs, which are designed to eliminate or inactivate self-reactive clones.37 In many autoimmune patients, these checkpoints are defective, allowing autoreactive B-cells to mature and expand.38
Therapeutic strategies are now being explored to correct these fundamental defects. For example, genetic studies have linked the protein tyrosine phosphatase PTPN22 to a higher risk for multiple autoimmune diseases. Research has demonstrated that inhibiting the enzymatic activity of PTPN22 can successfully reset defective central B-cell tolerance checkpoints in humanized mouse models, suggesting a potential pathway to prevent the generation of new autoreactive B-cells.38 Another novel approach is
B-cell delivered gene therapy. In this strategy, a patient's own B-cells are isolated and genetically modified ex vivo to express a tolerogenic form of the relevant autoantigen (e.g., fused to a non-inflammatory IgG backbone). When these engineered B-cells are re-infused into the patient, they function as tolerogenic APCs. They present the autoantigen to T-cells in a non-inflammatory context, which promotes the induction of Tregs specific for that antigen. These newly generated Tregs can then suppress the autoimmune response in a targeted manner.39 This approach aims to re-establish a dominant state of antigen-specific tolerance, offering the potential for a long-lasting, self-regulating cure.
Neutralizing Pathogenic Cytokine Networks to Restore Equilibrium
Beyond targeting the cellular architects of immunity, a powerful strategy for re-calibrating the immune system involves directly intercepting their molecular language. Cytokines are small proteins that act as potent signaling molecules, orchestrating the complex interactions between immune cells. They are the primary mediators of inflammation, dictating the nature, intensity, and duration of an immune response. In healthy individuals, cytokine production is tightly regulated, but in autoimmune and inflammatory diseases, this regulation breaks down, leading to the persistent and excessive production of pro-inflammatory cytokines. This "cytokine storm" drives chronic inflammation, recruits destructive immune cells, and causes progressive tissue damage. Therapeutic strategies aimed at neutralizing these key pathogenic cytokines or blocking their receptors have become a pillar of modern immunomodulation, offering a direct method to break the vicious cycle of inflammation and restore a more balanced immune state.
Key Inflammatory Axes in Autoimmunity
While the cytokine network is vast and complex, research has identified several key axes that play a dominant, pathogenic role in specific autoimmune diseases. Targeting these central hubs can have a profound impact on the overall inflammatory state.7
The TNF-α Axis: Tumor Necrosis Factor-alpha (TNF-α) is a master regulator of inflammation and a pivotal cytokine in the pathogenesis of diseases like rheumatoid arthritis (RA), psoriatic arthritis, and inflammatory bowel disease (IBD).42 Produced primarily by macrophages and T-cells, TNF-
α exists in two forms: a membrane-bound precursor (transmembrane TNF-α) and a soluble form that is cleaved and released into circulation.44 Both forms are biologically active and signal through two distinct receptors, TNF-R1 and TNF-R2. This signaling triggers a cascade of downstream events, including the induction of other pro-inflammatory cytokines like IL-1 and IL-6, the expression of adhesion molecules on endothelial cells to promote immune cell trafficking, and the activation of enzymes that degrade tissue, such as matrix metalloproteinases.43The IL-6 Axis: Interleukin-6 (IL-6) is a highly pleiotropic cytokine with a broad range of functions in immunity, inflammation, and hematopoiesis.46 In the context of diseases like RA, its effects are predominantly pro-inflammatory. Elevated levels of IL-6 are found in the serum and synovial fluid of RA patients, where it contributes to synovial inflammation, joint destruction, and the systemic manifestations of the disease, such as fatigue, anemia, and the production of acute-phase proteins like C-reactive protein (CRP) by the liver.47 IL-6 signals through a unique receptor system involving both a specific IL-6 receptor (IL-6R) and a ubiquitous signal-transducing subunit, gp130, allowing it to act on a wide variety of cell types.47
The IL-23/IL-17 Axis: This axis is now understood to be a distinct and critical pathway, particularly in diseases affecting barrier tissues like the skin and gut, such as psoriasis, psoriatic arthritis, and ankylosing spondylitis.49 In this pathway, IL-23, produced by dendritic cells and macrophages, is crucial for the expansion and maintenance of a subset of T-helper cells known as Th17 cells.8 These Th17 cells are the primary source of IL-17A, a potent pro-inflammatory cytokine. IL-17A acts on various stromal cells, including keratinocytes in the skin and fibroblasts in the synovium, inducing them to produce a range of inflammatory mediators, chemokines that recruit neutrophils, and antimicrobial peptides. This cascade drives the characteristic features of these diseases, such as the hyperproliferation of keratinocytes in psoriatic plaques.50 The distinction between this axis and the TNF/IL-6 axis provides a clear molecular basis for why therapies targeting these different pathways exhibit unique clinical efficacy profiles in different diseases. For example, the dramatic success of IL-17 inhibitors in psoriasis highlights the central role of this specific pathway in that particular pathology.
Mechanisms of Action: Blocking Cytokine Signaling
Therapeutic agents designed to inhibit these pathogenic cytokine axes primarily work through two distinct mechanisms to prevent the cytokine from delivering its signal to the target cell.16
Direct Neutralization: The most common approach utilizes high-affinity monoclonal antibodies that bind directly to the soluble cytokine in circulation. This forms an inert complex that prevents the cytokine from engaging with its cell surface receptor. Examples include infliximab and adalimumab, which neutralize TNF-α; secukinumab and ixekizumab, which neutralize IL-17A; and siltuximab, which neutralizes IL-6.16
Receptor Blockade: An alternative strategy is to target the cytokine receptor itself. Monoclonal antibodies are designed to bind to a specific subunit of the receptor complex, acting as a competitive antagonist that physically blocks the cytokine from docking and initiating a signal. This approach is exemplified by tocilizumab and sarilumab, which block the IL-6 receptor, and brodalumab, which blocks the IL-17 receptor A subunit.51
Regardless of the specific mechanism, the end result is the interruption of the downstream intracellular signaling cascades that propagate the inflammatory response. The TNF-α and IL-17 pathways predominantly rely on the activation of the Nuclear Factor-κB (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) signaling pathways. These are master regulators of gene expression, and their activation leads to the transcription of hundreds of pro-inflammatory genes.43 In contrast, the IL-6 and IL-23 pathways primarily signal through the
Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway, particularly activating STAT3. STAT3 is a critical transcription factor for the differentiation and function of pathogenic Th17 cells.45 By blocking these initial receptor-ligand interactions, anti-cytokine therapies effectively silence these powerful downstream inflammatory programs.
Challenges and Nuances in Cytokine Blockade
While cytokine-targeted therapies have transformed the treatment of many inflammatory diseases, their application is not without challenges and complexities. These therapies do not simply "suppress" the immune system; they strategically "re-wire" it by removing a key signaling hub. This can lead to both profound therapeutic benefits and unpredictable, non-linear network effects.
Network Redundancy and Combination Therapy: The immune system has built-in redundancy, with multiple cytokines often capable of performing similar functions. This means that blocking a single cytokine may be insufficient to control inflammation in some patients, as other pathways can compensate. This may explain the subset of patients who are non-responders to monotherapy and has spurred the development of bispecific antibodies or combination therapies that can simultaneously block two distinct pathways, such as TNF-α and IL-17A, which have shown enhanced efficacy in preclinical models.43
Differential Effects of Agents: Even within the same class, different drugs can have distinct biological effects. For example, anti-TNF agents vary in their ability to neutralize transmembrane TNF-α versus soluble TNF-α. Agents like infliximab are more potent at eliminating cells expressing transmembrane TNF-α, which is thought to be critical for their efficacy in granulomatous diseases like Crohn's disease, a setting where the agent etanercept is ineffective. This difference also influences their side-effect profiles, particularly the risk of reactivating granulomatous infections like tuberculosis.44
Paradoxical Effects: The complexity of the cytokine network can lead to counterintuitive outcomes. Under certain conditions, a cytokine-neutralizing antibody can form an immune complex with its target cytokine that, instead of being cleared, actually stabilizes the cytokine and prolongs its half-life. This can lead to a paradoxical enhancement of signaling over time, an "agonistic effect" that may contribute to a lack of efficacy.56 Furthermore, blocking one pro-inflammatory cytokine can sometimes tilt the immune balance in an unexpected direction. Anti-TNF-α therapy, for instance, has been associated with the development of drug-induced lupus-like syndromes. This may occur because TNF-α blockade can also lead to a reduction in the production of regulatory cytokines like IL-10, inadvertently shifting the immune system toward an autoimmune phenotype.57 These phenomena highlight that therapeutic intervention is not simple antagonism but a complex network modulation, requiring a deeper, systems-level understanding to predict and control its outcomes.
Augmenting Endogenous Regulatory Pathways for Durable Tolerance
The therapeutic strategies discussed thus far—reversing T-cell exhaustion, depleting autoreactive B-cells, and neutralizing pro-inflammatory cytokines—are primarily focused on inhibiting or eliminating the pathological components of an aberrant immune response. While highly effective, they represent an approach of "inhibiting the negative." An alternative and potentially more profound strategy is to "promote the positive" by augmenting the body's own endogenous pathways for maintaining immune tolerance. This approach is centered on Regulatory T-cells (Tregs), the master controllers of immune homeostasis. By enhancing the number or function of these specialized cells, it may be possible to re-establish a self-regulating state of tolerance, moving beyond chronic suppression toward a restorative medicine that offers the potential for durable, drug-free remission.
The Central Role of Regulatory T-Cells (Tregs) in Self-Tolerance
Tregs are a specialized subset of CD4$^{+}$ T-cells, distinguished by the expression of the master transcription factor Foxp3, whose non-negotiable function is to maintain immune homeostasis and enforce self-tolerance.2 They are the dedicated peacekeepers of the immune system. A deficiency in their number or a compromise in their function is a key factor in the pathogenesis of numerous autoimmune diseases, where the effector arms of the immune system are allowed to run rampant.58
Tregs employ a diverse arsenal of mechanisms to exert their suppressive effects. They can directly inhibit the function of effector T-cells and other immune cells through cell-to-cell contact, using surface molecules like CTLA-4 to deliver inhibitory signals. They also secrete a suite of powerful anti-inflammatory cytokines, including IL-10, TGF-β, and IL-35, which create a suppressive local microenvironment.58 Furthermore, Tregs express exceptionally high levels of the high-affinity IL-2 receptor, CD25. This allows them to act as a "cytokine sink," effectively consuming the available IL-2 in a microenvironment, thereby starving nearby effector T-cells of this essential growth factor and limiting their proliferation.58
Therapeutic Enhancement of Treg Function
Given their central role in maintaining tolerance, enhancing Treg function represents a highly attractive therapeutic strategy for autoimmune diseases. Several approaches are being actively pursued in clinical and preclinical settings.
Low-Dose IL-2 Therapy: The unique biology of Tregs makes them exquisitely sensitive to the cytokine IL-2. While high doses of IL-2 are immunostimulatory and can activate effector T-cells and NK cells, low doses can selectively promote the survival, expansion, and function of the Treg population due to their constitutive high-level expression of CD25.59 This creates a therapeutic window where a carefully titrated dose of IL-2 can be administered to specifically boost the regulatory arm of the immune system without significantly activating the inflammatory arm. Low-dose IL-2 therapy has shown promise in clinical trials for several autoimmune conditions, including lupus and graft-versus-host disease.59
Adoptive Cell Transfer of Polyclonal Tregs: A more direct approach involves isolating Tregs from a patient's peripheral blood, expanding them to large numbers in the laboratory using stimuli like anti-CD3/CD28 beads and high-dose IL-2, and then re-infusing this expanded, suppressive cell population back into the patient.58 This strategy of "Treg therapy" has been tested in numerous clinical trials for conditions ranging from type 1 diabetes to organ transplant rejection, with the goal of overwhelming the autoimmune response with a large force of regulatory cells.
Engineered Tregs for Antigen-Specific, Localized Immunosuppression
While polyclonal Treg therapies are promising, they lack antigen specificity and thus may exert off-target immunosuppressive effects. The next frontier in Treg therapy involves genetically engineering these cells to direct their potent suppressive activity exclusively to the site of autoimmune inflammation. This leverages the same core technologies that have revolutionized immuno-oncology, but repurposes them for a completely different therapeutic outcome. The modularity of modern immunotherapy platforms is on full display here; the same Chimeric Antigen Receptor (CAR) construct developed to guide a cytotoxic T-cell to kill a cancer cell can be placed into a different cellular "chassis"—a Treg—to guide it to suppress an autoimmune response.
CAR-Tregs: In this approach, Tregs are engineered to express a CAR that recognizes a specific antigen present on the target tissue under autoimmune attack. For example, in a patient with type 1 diabetes, Tregs could be engineered with a CAR that recognizes an antigen on pancreatic islet cells. When infused, these CAR-Tregs would traffic to the pancreas and, upon recognizing their target, become activated and deliver their full suppressive payload locally, protecting the islets from destruction by effector T-cells without causing systemic immunosuppression.4 This strategy is being actively investigated in clinical trials to promote tolerance to transplanted organs, where CAR-Tregs are designed to recognize molecules on the donor graft.21
TCR-Tregs: An alternative engineering strategy is to equip Tregs with a specific T-Cell Receptor (TCR) that recognizes a known autoantigen peptide presented by MHC molecules on APCs. This allows the engineered Tregs to engage with and regulate the very first steps of the autoimmune response in the draining lymph nodes. TCR-Treg therapy has shown remarkable efficacy in preclinical models of multiple sclerosis and rheumatoid arthritis, demonstrating its potential for highly targeted and potent immune regulation.58
These engineered Treg therapies represent a paradigm shift. They offer the potential for a "living drug" that can specifically home to the site of disease, deliver localized and powerful immunosuppression, and persist long-term, potentially inducing a durable, antigen-specific state of tolerance that could amount to a functional cure.
The Next Frontier: Systemic and Metabolic Interventions
The future of immune re-calibration extends beyond the direct manipulation of immune cells and their signaling pathways. Emerging research has illuminated two vast and interconnected frontiers that systemically regulate immune function: the metabolic programming of immune cells (immunometabolism) and the complex ecosystem of microbes residing in the gut (the microbiome-immune axis). These fields reveal that the functional state of the immune system is profoundly influenced by the bioenergetic status of its cells and by environmental inputs translated through our microbial partners. Targeting these systemic regulators offers a novel and holistic approach to restoring immune homeostasis, moving from cell-centric therapies to strategies that shape the entire immune environment.
Immunometabolism: Targeting Cellular Bioenergetics to Shape Immune Cell Fate
It is now clear that cellular metabolism is not merely a housekeeping function that provides energy and building blocks; it is an active and instructive layer of immune regulation. The functional fate of an immune cell is inextricably linked to its metabolic programming.60 A fundamental principle of immunometabolism is the "metabolic switch" that T-cells undergo upon activation.
Naïve and memory T-cells, which are in a quiescent state, primarily rely on efficient, catabolic metabolic pathways like oxidative phosphorylation (OXPHOS) and fatty acid oxidation (FAO) to generate ATP for long-term survival.60
Upon encountering an antigen, effector T-cells must undergo rapid proliferation and produce large quantities of cytokines. To meet these immense biosynthetic demands, they switch to a state of aerobic glycolysis, a less efficient but much faster pathway for generating energy and the carbon backbones needed for anabolic processes like nucleotide and lipid synthesis.60
Regulatory T-cells (Tregs), in contrast, often maintain a more oxidative metabolic profile, favoring FAO to support their sustained suppressive function and survival in nutrient-poor environments.60
This clear metabolic dichotomy between pro-inflammatory effector cells (glycolytic) and anti-inflammatory regulatory cells (oxidative) creates a powerful therapeutic window. It becomes possible to modulate immune responses by targeting metabolic enzymes and pathways rather than traditional immune receptors. This represents a completely new axis for therapeutic intervention.
In the context of autoimmunity, where hyperactive, glycolytic effector T-cells drive pathology, the strategy is to inhibit their metabolic engine. Drugs like metformin, a first-line treatment for type 2 diabetes, can inhibit mitochondrial complex I, and 2-deoxy-D-glucose (2-DG) directly inhibits glycolysis. By limiting the fuel available for aerobic glycolysis, these agents can selectively dampen the function and proliferation of pathogenic effector T-cells, while having less of an impact on the oxidative metabolism of Tregs.60 Conversely, drugs that activate transcription factors like PPARs can actively promote a shift towards FAO, pushing T-cells towards a more regulatory or memory-like phenotype.60
In cancer, the logic is inverted. The tumor microenvironment is often a harsh, nutrient-depleted landscape. Tregs, with their flexible, oxidative metabolism, are well-adapted to thrive in this environment, where they suppress anti-tumor immunity. Glycolytic effector T-cells, however, are at a metabolic disadvantage. Therefore, a key therapeutic strategy is to target the metabolic pathways that support Treg function. For example, blocking the fatty acid transporter CD36, which is highly expressed on tumor-infiltrating Tregs, can selectively impair their function and enhance the efficacy of anti-tumor T-cells.58
The Microbiome-Immune Axis: A Systemic Regulator
The immune system does not develop or function in a sterile vacuum. It is in constant dialogue with the trillions of microbes that inhabit the gut. This vast microbial community, or microbiome, functions almost as a metabolic "organ," playing a critical role in educating, shaping, and regulating host immunity from birth.9 An imbalance in this microbial community, known as dysbiosis, has been strongly linked to a wide range of immune-mediated diseases, including IBD, allergies, and several autoimmune conditions.9
The microbiome acts as a systemic rheostat for immune homeostasis, translating external environmental inputs, most notably diet, into potent internal immunoregulatory signals. The primary mechanism for this modulation is the production of microbial metabolites.9 Gut bacteria ferment dietary fibers—complex carbohydrates that are indigestible by the host—into a range of bioactive molecules, most prominently short-chain fatty acids (SCFAs) like butyrate, propionate, and acetate.9 These SCFAs are absorbed into circulation and exert powerful, systemic immunomodulatory effects. They are known to directly promote the differentiation and suppressive function of Tregs, enhance the integrity of the intestinal epithelial barrier to prevent the leakage of inflammatory stimuli, and have direct anti-inflammatory effects on various immune cells.2
This understanding provides a strong scientific rationale for therapeutic interventions aimed at modulating the microbiome to restore immune balance.
Probiotics, Prebiotics, and Synbiotics: The administration of live beneficial bacteria (probiotics), the non-digestible fibers they consume (prebiotics), or a combination of the two (synbiotics) are strategies designed to shift the composition of the gut microbiome toward a more balanced, anti-inflammatory state.10
Fecal Microbiota Transplantation (FMT): In some cases, a more drastic "reset" of the microbial ecosystem can be achieved by transferring the entire fecal microbiota from a healthy donor to a patient.
Targeted Metabolite Delivery: As our understanding of specific microbial functions grows, more precise strategies are being developed. These include advanced oral delivery systems designed to transport specific beneficial metabolites, like butyrate, directly to the lower gastrointestinal tract where they can exert their maximal immunomodulatory effects.65
By viewing the microbiome as a key intermediary that translates lifestyle into molecular signals that calibrate immune tone, these approaches are moving from the realm of general wellness into the forefront of precision immunomodulatory medicine.
Synthesis and Future Perspectives
The field of immunotherapy is undergoing a profound transformation, moving decisively away from the blunt instrument of broad immunosuppression toward a diverse and sophisticated toolkit for precision immune re-calibration. The strategies of reinvigorating exhausted T-cells, selectively depleting pathogenic B-cells, neutralizing key cytokine networks, and augmenting endogenous regulatory pathways represent a collective shift towards therapies that are more specific, more effective, and potentially curative. As we look to the future, the integration of these approaches with systemic modulators like immunometabolic agents and microbiome-based therapies promises to usher in an era of truly personalized medicine for immune-mediated diseases. However, realizing this promise requires confronting the substantial challenges of therapeutic resistance, toxicity, and patient stratification that arise from the inherent complexity of the immune system itself.
A Comparative Framework for Targeted Immunotherapies
The various therapeutic modalities discussed each offer a unique approach to restoring immune homeostasis, with distinct mechanisms, levels of specificity, and clinical applications. Immune checkpoint inhibitors (ICIs) work by releasing the brakes on pre-existing T-cells, a powerful strategy for cancer but one that risks systemic autoimmunity.18 In contrast, cellular therapies like CAAR-T cells offer the ultimate in precision for autoantibody-driven diseases by surgically removing the specific pathogenic B-cell clone.21 Cytokine blockers provide a more targeted way to quell inflammation than traditional DMARDs but must contend with the redundancy and complexity of the cytokine network.67 Treg-based therapies, particularly engineered CAR-Tregs, represent a restorative approach, aiming to re-establish dominant, antigen-specific tolerance rather than simply depleting pathogenic cells.22 Finally, metabolic and microbial interventions offer a way to modulate the entire immune environment, shaping cell fate and function in a systemic manner.65
The optimal therapeutic strategy is dictated by the underlying pathophysiology of the disease.69 An autoantibody-mediated disease like pemphigus vulgaris is an ideal candidate for a CAAR-T approach that eliminates the source of the antibody. A T-cell-driven disease with a known autoantigen, like multiple sclerosis, may be better suited for an antigen-specific Treg therapy. A disease characterized by a "cytokine storm" may benefit most from targeted cytokine blockade. The future of treatment will involve a deep biological understanding of a patient's specific disease to match them with the most appropriate tool from this expanding therapeutic toolbox.
Overcoming Key Challenges in Precision Immunotherapy
Despite their immense promise, the widespread application of these advanced therapies is hindered by several key challenges, all of which are direct consequences of the dynamic complexity and heterogeneity of the immune system and the diseases it causes.
Therapeutic Resistance: Both primary (initial non-response) and acquired (relapse after response) resistance are major obstacles.41 In cancer, this can be driven by the immense genetic heterogeneity within a tumor, where some subclones may lack the target antigen or develop mutations in pathways that make them invisible to the immune system.20 In autoimmunity, the phenomenon of epitope spreading can render a highly specific therapy ineffective over time as the immune response broadens to target new self-antigens.34 The redundancy of immune pathways also contributes; blocking one signaling node can lead to compensatory upregulation of another.70 The most promising strategy to combat resistance is the rational design of combination therapies that target multiple, non-overlapping mechanisms simultaneously, making it harder for the disease to escape.41
Toxicity Management: Targeted does not mean non-toxic. The pathways targeted by precision immunotherapies are often integral to normal physiological processes, leading to unique on-target, off-tumor toxicities.20 The irAEs caused by ICIs are a direct result of disrupting self-tolerance, and the cytokine release syndrome (CRS) and neurotoxicity seen with CAR T-cell therapies are a consequence of massive immune activation.20 Mitigating these toxicities requires better predictive biomarkers, sophisticated patient monitoring, and the engineering of next-generation cellular therapies with built-in safety switches or tunable activity.4
Patient Stratification and Biomarkers: Perhaps the greatest unmet need is the ability to reliably predict which patients will respond to a given therapy and who is at highest risk for severe toxicity.71 Current biomarkers, such as PD-L1 expression for ICIs, have proven to be inconsistent and insufficient because they capture only a single, static snapshot of a vast and dynamic biological network.20 A truly personalized approach requires the development of robust, multi-omic biomarkers that can integrate information from the patient's genomics, proteomics, tumor microenvironment, and microbiome to create a comprehensive picture of their individual disease state and guide therapy selection.71
The Trajectory Towards Personalized Immune Re-calibration
The trajectory of the field is clear: it is moving inexorably towards combinatorial, personalized regimens designed to induce durable immune tolerance. The one-size-fits-all, chronic treatment model is giving way to strategies that aim for a definitive immune reset. Cellular therapies, with their potential for a single administration to induce long-term, drug-free remission, represent the vanguard of this curative-intent approach.21
The ultimate vision for the future involves an integrated, multi-layered strategy. A patient with an autoimmune disease might first undergo deep biomarker profiling to identify the specific cellular driver (e.g., an autoreactive B-cell clone), the dominant inflammatory pathway (e.g., the IL-17 axis), and any systemic imbalances (e.g., dysbiosis or a pro-inflammatory metabolic state). Treatment could then involve a highly specific cellular therapy (e.g., CAAR-T) to eliminate the pathogenic clone, combined with a transient course of a cytokine blocker to quell initial inflammation. This would be supported by systemic interventions, such as a tailored diet or prebiotic supplements to modulate the microbiome and a metabolic drug to favor a regulatory immune environment. This holistic approach, which combines direct immune cell manipulation with the optimization of the metabolic and microbial context, represents the pinnacle of personalized immune re-calibration—a therapy designed not just to treat a disease, but to restore the elegant and essential balance of immune homeostasis.
Acknowledgement
I acknowledge the use of Gemini AI in the preparation of this report. Specifically, it was used to: (1) brainstorm and refine the initial research questions; (2) assist in writing and debugging Python scripts for statistical analysis; and (3) help draft, paraphrase, and proofread sections of the final manuscript. I reviewed, edited, and assume full responsibility for all content.
Works cited
(PDF) Failure of Immune Homeostasis - The Consequences of Under and Over Reactivity, accessed September 11, 2025, https://www.researchgate.net/publication/7394967_Failure_of_Immune_Homeostasis_-_The_Consequences_of_Under_and_Over_Reactivity
Immune homeostasis - (Immunobiology) - Vocab, Definition, Explanations | Fiveable, accessed September 11, 2025, https://library.fiveable.me/key-terms/immunobiology/immune-homeostasis
Immunomodulation: precision targeting for restoring immune ..., accessed September 11, 2025, https://portlandpress.com/essaysbiochem/article/69/02/19/236516/Immunomodulation-precision-targeting-for-restoring
What are the different types of drugs available for Tregs cell therapy? - Patsnap Synapse, accessed September 11, 2025, https://synapse.patsnap.com/article/what-are-the-different-types-of-drugs-available-for-tregs-cell-therapy
Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus - MDPI, accessed September 11, 2025, https://www.mdpi.com/1422-0067/25/14/7726
The Role of PD-1 in Acute and Chronic Infection - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.00487/full
Cytokine regulation of immune tolerance | Burns & Trauma - Oxford Academic, accessed September 11, 2025, https://academic.oup.com/burnstrauma/article/2/1/2321-3868.124771/5650497
Molecular Mechanisms of Immune Regulation: A Review - MDPI, accessed September 11, 2025, https://www.mdpi.com/2073-4409/14/4/283
From Microbes to Immunity: A Comprehensive Review of ..., accessed September 11, 2025, https://jhrlmc.com/index.php/home/article/download/238/234
The Role of the Microbiome in Immune System Modulation - Research and Reviews, accessed September 11, 2025, https://www.rroij.com/open-access/the-role-of-the-microbiome-in-immune-system-modulation.pdf
Disruption of Homeostasis - Advanced | CK-12 Foundation, accessed September 11, 2025, https://flexbooks.ck12.org/cbook/ck-12-advanced-biology/section/17.6/primary/lesson/disruption-of-homeostasis-advanced-bio-adv/
Immunomodulators: Types, Uses, Effectiveness, Side Effects, More - Healthline, accessed September 11, 2025, https://www.healthline.com/health/immunomodulators
Immunosuppressants: Definition, Uses & Side Effects - Cleveland Clinic, accessed September 11, 2025, https://my.clevelandclinic.org/health/treatments/10418-immunosuppressants
The Role of PD-1 in Acute and Chronic Infection - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7105608/
Exploring Immunomodulation to Balance Maladaptive Inflammation and Restore Tissue Homeostasis - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/research-topics/63511/exploring-immunomodulation-to-balance-maladaptive-inflammation-and-restore-tissue-homeostasis
Recent Progress in Capturing and Neutralizing Inflammatory Cytokines | CCS Chemistry, accessed September 11, 2025, https://www.chinesechemsoc.org/doi/10.31635/ccschem.020.202000165
Everything You Need to Know about Immunosuppressants - Arthritis Foundation, accessed September 11, 2025, https://www.arthritis.org/drug-guide/medication-topics/immunosuppressant
Immunotherapy in Autoimmune Diseases: A Review of Recent Breakthroughs in Using Immunotherapy for Conditions Like Rheumatoid Arthritis, Lupus, and Multiple Sclerosis, accessed September 11, 2025, https://ijmps.com/article/immunotherapy-in-autoimmune-diseases-a-review-of-recent-breakthroughs-in-using-immunotherapy-for-conditions-like-rheumatoid-arthritis-lupus-and-multiple-sclerosis-43/
PD-1/PD-L1 Inhibitors in Patients With Preexisting Autoimmune Diseases - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8971753/
Biomarkers in Precision Cancer Immunotherapy: Promise and Challenges, accessed September 11, 2025, https://ascopubs.org/doi/10.1200/EDBK_280571
Current advancements in cellular immunotherapy for autoimmune ..., accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11739237/
CAR-Based Therapy for Autoimmune Diseases: A Novel Powerful Option - MDPI, accessed September 11, 2025, https://www.mdpi.com/2073-4409/12/11/1534
Adoptive Cell Transfer: Monitor T Cell Exhaustion - R&D Systems, accessed September 11, 2025, https://www.rndsystems.com/product-highlights/adoptive-cell-transfer-monitor-t-cell-exhaustion
T Cell Exhaustion - ResearchGate, accessed September 11, 2025, https://www.researchgate.net/publication/377102768_T_Cell_Exhaustion
Characterization of Exhausted T Cell Signatures in Pan-Cancer Settings - ResearchGate, accessed September 11, 2025, https://www.researchgate.net/publication/389594941_Characterization_of_Exhausted_T_Cell_Signatures_in_Pan-Cancer_Settings
Exhausted T cells hijacking the cancer-immunity cycle ... - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1151632/full
Study finds hallmarks of T cell exhaustion within hours of tumour exposure - ecancer, accessed September 11, 2025, https://ecancer.org/en/news/23478-study-finds-hallmarks-of-t-cell-exhaustion-within-hours-of-tumour-exposure
Emerging Role of PD-1/PD-L1 Inhibitors in Chronic Liver Diseases - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2021.790963/full
The role of PD-1 signaling in health and immune-related diseases - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1163633/full
When exhausted, cancer-fighting T cells may switch sides - Drug Target Review, accessed September 11, 2025, https://www.drugtargetreview.com/article/107506/when-exhausted-cancer-fighting-t-cells-may-switch-sides/
Clinical implications of T cell exhaustion for cancer immunotherapy - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10984554/
PD-1 Inhibitor Immune-Related Adverse Events in Patients With Preexisting Endocrine Autoimmunity | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic, accessed September 11, 2025, https://academic.oup.com/jcem/article/103/10/3589/5073238
Comparison of efficacy discrepancy between early-phase clinical trials and phase III trials of PD-1/PD-L1 inhibitors - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10806571/
Therapeutic Targeting of Autoreactive B Cells: Why, How, and When?, accessed September 11, 2025, https://www.mdpi.com/2227-9059/9/1/83
B Cells in Autoimmune Diseases - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3692299/
Can autoimmune disease be cured by deep CD19+ cell depletion? - Oxford Academic, accessed September 11, 2025, https://academic.oup.com/jimmunol/article/214/6/1075/8090055
B Cell Tolerance in Health and Disease - MDPI, accessed September 11, 2025, https://www.mdpi.com/2073-4468/3/1/116
Correcting defective early B cell tolerance checkpoints common to many autoimmune diseases | Meffre Lab | Stanford Medicine, accessed September 11, 2025, https://med.stanford.edu/meffre-lab/research/correcting-defective-early-B-cell-tolerance.html
B cell delivered gene therapy for tolerance induction: role of autoantigen-specific B cells, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2926203/
B cell depletion therapies in autoimmune diseases: Monoclonal antibodies or chimeric antigen receptor-based therapy? - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1126421/full
The Challenges in Fulfilling the Promise of Precision Oncology - Applied Clinical Trials, accessed September 11, 2025, https://www.appliedclinicaltrialsonline.com/view/challenges-fulfilling-promise-precision-oncology
Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4889878/
A native-like bispecific antibody suppresses the inflammatory cytokine response by simultaneously neutralizing tumor necrosis factor-alpha and interleukin-17A - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC5669854/
Transmembrane TNF-α: structure, function and interaction with anti ..., accessed September 11, 2025, https://academic.oup.com/rheumatology/article/49/7/1215/1785515
TNFα Promotes Th17 Cell Differentiation through IL-6 and IL-1β Produced by Monocytes in Rheumatoid Arthritis - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4243768/
What are IL-6 inhibitors and how do they work? - Patsnap Synapse, accessed September 11, 2025, https://synapse.patsnap.com/article/what-are-il-6-inhibitors-and-how-do-they-work
Understanding the Role of Interleukin-6 (IL-6) in the Joint and ..., accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7410942/
IL-6 inhibitor for the treatment of rheumatoid arthritis: A comprehensive review, accessed September 11, 2025, https://www.researchgate.net/publication/328948602_IL-6_inhibitor_for_the_treatment_of_rheumatoid_arthritis_A_comprehensive_review
Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective | Journal of Experimental Medicine | Rockefeller University Press, accessed September 11, 2025, https://rupress.org/jem/article/217/1/e20191123/132585/Targeting-interleukin-17-in-chronic-inflammatory
A Mechanistic Insight into the Pathogenic Role of Interleukin 17A in Systemic Autoimmune Diseases - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9129985/
Clinical and Molecular Effects of Interleukin-17 Pathway Blockade in Psoriasis - JDDonline, accessed September 11, 2025, https://jddonline.com/articles/clinical-and-molecular-effects-of-interleukin-17-pathway-blockade-in-psoriasis-S1545961620P0138X/
A Review of the Safety of Interleukin-17A Inhibitor Secukinumab - MDPI, accessed September 11, 2025, https://www.mdpi.com/1424-8247/15/11/1365
What is the mechanism of action of Secukinumab? - Patsnap Synapse, accessed September 11, 2025, https://synapse.patsnap.com/article/what-is-the-mechanism-of-action-of-secukinumab
Anti-IL-6 – Knowledge and References - Taylor & Francis, accessed September 11, 2025, https://taylorandfrancis.com/knowledge/Medicine_and_healthcare/Pharmaceutical_medicine/Anti-IL-6/
Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis - MDPI, accessed September 11, 2025, https://www.mdpi.com/1422-0067/23/2/859
A Generic Mechanism for Enhanced Cytokine Signaling via Cytokine-Neutralizing Antibodies | PLOS One - Research journals, accessed September 11, 2025, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0149154
Mechanisms of Tumor Necrosis Factor-Alpha Inhibitor-Induced Systemic Lupus Erythematosus - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2022.870724/full
Regulating the regulatory T cells as cell therapies in ... - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1244298/full
Cytokine Signaling in the Development and Homeostasis of Regulatory T cells - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC5830895/
New Developments in T Cell Immunometabolism and ... - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2022.914136/full
Emerging concepts in immunotherapy – T cell metabolism as a therapeutic target - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4990080/
Immunometabolism: A target for the comprehension of immune response toward transplantation - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6656658/
Immune System Modulations by Products of the Gut Microbiota - MDPI, accessed September 11, 2025, https://www.mdpi.com/2076-393X/8/3/461
The gut microbiome, immune modulation, and cognitive decline: insights on the gut-brain axis - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11794507/
Engineering strategies to modulate the gut microbiome and immune system - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10766079/
Innovative Approaches to Medical Rehabilitation: Regeneration, Homeostasis, and Microbiome Synergy - Preprints.org, accessed September 11, 2025, https://www.preprints.org/frontend/manuscript/a2daae1908e44f692c7965923824b5de/download_pub
Dual-target immunotherapies in NSCLC: a systematic review and meta-analysis of randomized clinical trials - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1605877/full
Immunometabolism at the Intersection of Signaling Networks and Therapeutic Strategies, accessed September 11, 2025, https://www.frontiersin.org/research-topics/70814/immunometabolism-at-the-intersection-of-signaling-networks-and-therapeutic-strategies
Advances in Antigen-Specific Immunotherapies for Autoimmune Disease Management, accessed September 11, 2025, https://www.frontiersin.org/research-topics/65974/advances-in-antigen-specific-immunotherapies-for-autoimmune-disease-management
Challenges of the Immunotherapy: Perspectives and Limitations of ..., accessed September 11, 2025, https://www.mdpi.com/1422-0067/23/5/2847
Biomarkers in Precision Cancer Immunotherapy: Promise and Challenges - ResearchGate, accessed September 11, 2025, https://www.researchgate.net/publication/341665197_Biomarkers_in_Precision_Cancer_Immunotherapy_Promise_and_Challenges
Advancing Precision Immunotherapy in Triple-Negative Breast Cancer Through Plasma Proteomics - Pharmacy Times, accessed September 11, 2025, https://www.pharmacytimes.com/view/advancing-precision-immunotherapy-in-triple-negative-breast-cancer-through-plasma-proteomics