Neurovascular Sequelae of SARS-CoV-2: A Comprehensive Analysis of Blood-Brain Barrier Disruption, Microthrombosis, and Cognitive Impairment in Long COVID
Long COVID brain fog arises from two synergistic pathologies: a leaky blood-brain barrier and persistent microthrombosis, causing chronic inflammation and cerebral hypoperfusion.
The Compromised Cerebral Fortress: Clinical and Imaging Evidence for Persistent Blood-Brain Barrier Disruption in Long COVID
The emergence of post-acute sequelae of SARS-CoV-2 infection (PASC), commonly known as long COVID, has presented a formidable challenge to the global medical and scientific communities. Among its most debilitating and prevalent manifestations are a constellation of neurological symptoms, including profound fatigue, memory deficits, and difficulties with concentration, collectively termed "brain fog".1 For a significant period, the biological underpinnings of these subjective complaints remained elusive, hindering the development of effective diagnostic and therapeutic strategies. However, a growing body of rigorous scientific investigation has begun to converge on the central nervous system's (CNS) vasculature as a primary site of pathology. Evidence now compellingly demonstrates that a core feature of neurological long COVID is a sustained, localized disruption of the blood-brain barrier (BBB), the highly selective border of endothelial cells that protects the delicate neural microenvironment. This section will systematically review the clinical and imaging evidence that establishes the extent, anatomical localization, and chronicity of BBB disruption post-COVID-19, linking this physical breach to the neurological deficits observed in patients. Through an analysis of advanced neuroimaging, circulating biomarkers, and clinical correlates, it becomes clear that the "leaky brain" is not a theoretical concept but a measurable and persistent pathology central to the pathophysiology of long COVID.
Characterizing the "Leaky Brain": Advanced Neuroimaging of BBB Permeability
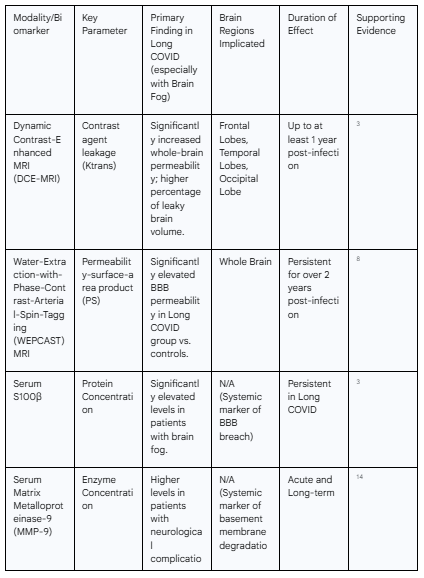
The direct, in vivo assessment of BBB integrity has been revolutionized by advanced neuroimaging techniques, which have provided the most definitive evidence for its compromise in long COVID. Standard clinical magnetic resonance imaging (MRI) scans of individuals with long COVID-associated brain fog are often unremarkable, showing no gross pathological changes such as lesions or hemorrhages that could explain the severity of their symptoms.3 This discrepancy highlights that the pathology is not one of macrostructural damage but of microvascular dysfunction, necessitating more sophisticated imaging modalities to detect.
The gold standard for this purpose has become dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). This technique involves the intravenous administration of a gadolinium-based contrast agent, a molecule that is normally excluded from the brain parenchyma by an intact BBB. By acquiring rapid, sequential images, DCE-MRI can track the spatiotemporal dynamics of the contrast agent's distribution. In a healthy brain, the agent remains within the vasculature; however, in the presence of a compromised BBB, it leaks into the surrounding tissue. The rate and extent of this leakage can be quantified to generate detailed, three-dimensional maps of BBB permeability.
Multiple independent studies utilizing DCE-MRI have consistently demonstrated significantly elevated whole-brain leakage in long COVID patients who report experiencing brain fog.3 When compared to individuals who also had long COVID but did not report brain fog, as well as to control subjects who had fully recovered from COVID-19, the brain fog cohort exhibits a quantitatively greater degree of BBB permeability.4 This is not a subtle effect; analyses have revealed a significantly higher percentage of total brain volume exhibiting leaky blood vessels in the brain fog group.3 This finding is crucial as it provides an objective, measurable biological correlate for a previously subjective clinical complaint. The subjective experience of cognitive difficulty is directly linked to a physical breach in the brain's protective barrier.
Further corroborating these findings, alternative, non-contrast MRI techniques have been developed to assess BBB permeability, avoiding the potential risks associated with gadolinium agents. One such method is water-extraction-with-phase-contrast-arterial-spin-tagging (WEPCAST) MRI. This technique uses magnetically labeled arterial blood water as an endogenous tracer. By measuring the rate at which this labeled water moves from the capillaries into the brain tissue, WEPCAST can estimate the permeability-surface-area product (PS), a quantitative measure of barrier transport. A cross-sectional study employing WEPCAST in a cohort of long COVID patients found that the PS value was significantly elevated in the long COVID group compared to recovered controls, even after adjusting for confounding variables like age and sex.8 The convergence of evidence from both contrast-based (DCE-MRI) and non-contrast (WEPCAST) methods strengthens the conclusion that increased BBB permeability is a robust and reproducible feature of long COVID.
Anatomical Localization: A Targeted Attack on Cognitive Centers
The disruption of the BBB in long COVID is not a diffuse, uniform phenomenon across the entire brain. Instead, region-of-interest (ROI) analyses from DCE-MRI studies have pinpointed specific neuroanatomical areas that are disproportionately affected. These analyses consistently reveal significantly higher levels of vascular leakage in the left and right temporal lobes and the left and right frontal cortex.3 This anatomical specificity is of profound importance, as these brain regions are the critical hubs for the very cognitive functions that are impaired in brain fog. The frontal lobes are the seat of executive functions, including planning, decision-making, working memory, and attentional control. The temporal lobes, particularly structures like the hippocampus, are essential for the formation and retrieval of memories.
The localization of maximal BBB leakage to these specific cognitive centers provides a direct mechanistic link between the observed vascular pathology and the clinical symptomology. The chronic influx of blood-borne molecules, immune cells, and inflammatory mediators into these precise regions can disrupt the delicate synaptic processes and neural network activity required for normal cognition.12 Furthermore, some studies have noted corresponding volumetric deficits in the frontal and temporal lobes of long COVID patients, suggesting that the persistent microvascular leakage and the ensuing neuroinflammatory environment may, over time, contribute to tangible structural changes and tissue loss in these vulnerable areas.3 This targeted assault on the brain's cognitive machinery explains why memory and executive function are so profoundly affected, transforming "brain fog" from a vague complaint into a predictable consequence of localized neurovascular injury.
Temporal Dynamics: Evidence for a Chronic, Sustained Dysfunction
A critical aspect of the BBB pathology in long COVID is its remarkable persistence. This is not merely a transient phenomenon confined to the acute phase of SARS-CoV-2 infection that slowly resolves. Instead, longitudinal and cross-sectional studies demonstrate a chronic, ongoing state of barrier dysfunction that can last for months and even years.
Initial evidence from the acute phase of COVID-19 showed that BBB disruption was present, particularly in patients with severe disease and neurological manifestations.6 However, follow-up imaging has revealed that this disruption persists long after the acute infection has cleared. DCE-MRI studies have documented enhanced BBB permeability in individuals with brain fog for up to one year after their initial positive PCR test.3 More recent and longer-term studies using WEPCAST MRI have extended this timeline even further, identifying subtle but statistically significant BBB disruption in long COVID patients more than two years after their initial infection.8
This chronicity is a key feature that distinguishes the pathophysiology of long COVID. It suggests that the initial viral infection triggers a self-sustaining pathological process within the cerebral vasculature that does not resolve with viral clearance from the respiratory system. This reframes the condition not as a protracted recovery but as an active, ongoing disease state. The underlying driver of this sustained insult is an area of intense investigation, but one potential explanation comes from postmortem studies of severe COVID-19 cases, which have found evidence of persistent viral RNA in multiple anatomical sites, including the brain, for up to 230 days after symptom onset.4 While these data are from the most severe cases, they raise the possibility that a persistent viral reservoir or lingering viral proteins within the CNS could provide a continuous stimulus for endothelial dysfunction and inflammation, thereby perpetuating the BBB leakiness for years.15
Clinical Correlates: Linking BBB Permeability to Neurological Symptoms
The most compelling evidence for the clinical relevance of BBB disruption is the direct correlation between the degree of vascular leakage and the presence and severity of neurological symptoms. As noted, multiple studies have stratified long COVID cohorts based on the self-reported presence or absence of brain fog. In these comparisons, the individuals with brain fog consistently and significantly exhibit greater BBB permeability than their counterparts without brain fog, as well as recovered controls.3 This robust association establishes BBB dysfunction as a key pathophysiological feature specifically linked to cognitive impairment.
However, the relationship between BBB permeability and the full spectrum of neurological symptoms is complex and nuanced. A large study utilizing the non-contrast WEPCAST MRI technique made a particularly intriguing discovery. While it confirmed that the long COVID group had significantly higher overall BBB permeability, a more detailed analysis within this group found that higher permeability (elevated PS values) was significantly associated with poorer motor function. Surprisingly, this same study did not find a statistically significant correlation between BBB permeability and performance on other cognitive domains, such as memory or executive function.8
This dissociation is highly informative. It suggests that "brain fog" is likely a multifactorial syndrome, with different underlying mechanisms potentially driving different symptom clusters. The pathway from a leaky BBB to neuronal dysfunction is thought to be primarily mediated by neuroinflammation—the influx of immune cells and inflammatory molecules that disrupt synaptic function.16 It is plausible that motor control pathways are particularly sensitive to this type of inflammatory insult, thus explaining the strong correlation. Conversely, other cognitive deficits, such as those related to attention and processing speed, may be more dependent on another key pathology seen in long COVID: cerebral microthrombosis and the resulting chronic hypoperfusion.12 This possibility, which will be explored in detail in Section 3, indicates that a comprehensive understanding of neurological long COVID requires considering at least two parallel, interacting vascular pathologies. The leaky BBB may be a primary driver of inflammation-related symptoms like motor deficits, while microvascular occlusion may be more central to ischemia-related cognitive symptoms.
Circulating Biomarkers as Windows into Neurovascular Injury
Complementing the advanced imaging data, the analysis of blood-based biomarkers provides accessible and quantitative biochemical evidence of the ongoing injury to the neurovascular unit. The presence of brain-specific proteins in the peripheral circulation is a direct indicator of a breach in the BBB, while other markers can illuminate the specific pathological processes, such as enzymatic degradation of the vascular structure or glial cell activation.
S100β: This protein is predominantly produced by astrocytes within the CNS and is normally found at very low concentrations in the blood. Its presence in serum is a well-established and sensitive marker of BBB disruption. In studies of long COVID, patients with brain fog have been found to have significantly higher serum levels of S100β compared to both COVID patients without brain fog and uninfected controls.3 This finding provides powerful biochemical confirmation that the barrier is physically compromised, allowing brain-derived proteins to leak out into the periphery.
Matrix Metalloproteinase-9 (MMP-9): MMP-9 is a proteolytic enzyme that plays a key role in remodeling the extracellular matrix. One of its primary targets is type IV collagen, a critical structural component of the vascular basement membrane that underpins the BBB. In COVID-19 patients with neurological complications, blood levels of MMP-9 are elevated, particularly during the acute phase of infection.14 Moreover, its levels show a severity dependency in the long-term, suggesting that sustained MMP-9 activity could be contributing to the chronic degradation of BBB integrity.14
Glial Fibrillary Acidic Protein (GFAP): GFAP is an intermediate filament protein that is highly specific to astrocytes. Its elevation in the blood is indicative of astrogliosis, a reactive state of astrocytes that occurs in response to CNS injury, including neuroinflammation and ischemia. Increased GFAP levels have been observed in COVID-19 patients, signaling that the vascular distress is triggering a reactive response from the glial cells that are intimately associated with the BBB.11
Neurofilament Light Chain (NFL): NFL is a structural protein of the neuronal cytoskeleton. When neurons are damaged or die, NFL is released into the cerebrospinal fluid and subsequently into the bloodstream. Elevated blood levels of NFL are therefore a direct marker of axonal injury. The finding that NFL levels are high in COVID-19 patients with neurological symptoms confirms that the upstream vascular pathology—the leaky BBB and associated inflammation—is ultimately translating into downstream damage to the neurons themselves.12
Together, this panel of biomarkers paints a coherent picture of the pathological cascade. S100β and MMP-9 confirm the physical and enzymatic breakdown of the barrier, GFAP indicates the reactive response of perivascular astrocytes, and NFL provides the ultimate proof of consequent neuronal injury.
Table 1: Summary of Neuroimaging and Biomarker Findings in Long COVID
The Molecular Cascade of SARS-CoV-2-Induced Endotheliopathy and BBB Breakdown
The clinical and imaging evidence for a persistent, leaky BBB in long COVID necessitates a deep investigation into the underlying molecular mechanisms. The breakdown of this sophisticated biological barrier is not a single event but the culmination of a multi-pronged and sustained assault on the neurovascular unit—the intricate system of endothelial cells, pericytes, astrocytes, and the basement membrane. This assault is orchestrated through several parallel and interacting pathways: direct viral infection of the barrier's cellular components, the overwhelming systemic inflammatory response, targeted immune-mediated damage, and the enzymatic deconstruction of the barrier's structural foundations. Understanding this complex molecular cascade is essential for identifying specific therapeutic targets to restore vascular integrity and alleviate the neurological sequelae of the disease.
Direct Viral Insult: The Virus at the Gates
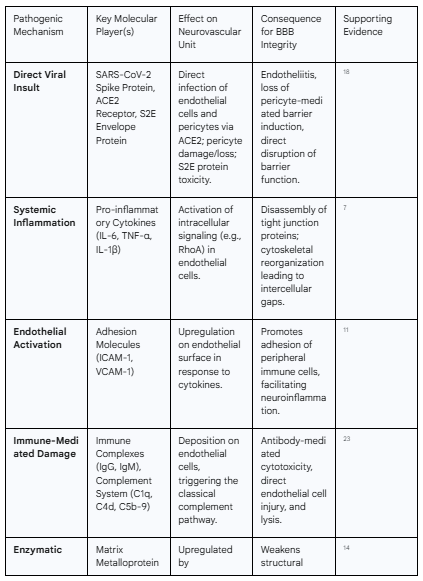
The initial trigger for neurovascular injury in many cases is the direct interaction of the SARS-CoV-2 virus with the cells of the BBB itself. The primary portal for viral entry into host cells is the Angiotensin-Converting Enzyme 2 (ACE2) receptor, which the viral spike protein binds with high affinity.18 Crucially, ACE2 is expressed on the key cellular constituents of the neurovascular unit, including cerebral endothelial cells, which form the wall of the barrier, and pericytes, the mural cells that ensheath the capillaries and are critical for inducing and maintaining their barrier properties.18 This expression pattern makes the BBB a direct target for the virus.
Upon binding to ACE2, the virus can infect these cells, initiating a cascade of local pathological events. Direct infection of endothelial cells leads to a state known as endotheliitis, or inflammation of the endothelium, which promotes a pro-inflammatory and pro-thrombotic cellular phenotype.19 Infected pericytes undergo significant damage, and in some cases, are lost entirely.20 The loss of pericytes is particularly detrimental, as these cells are essential for signaling to endothelial cells to form and maintain robust tight junctions and regulate blood flow. Their depletion results in a destabilized, leaky, and immature vascular network, directly contributing to increased BBB permeability.21
Furthermore, the pathogenic effects are not solely dependent on successful viral replication within the CNS. Individual viral components can act as potent toxins to the neurovascular unit. Research on the SARS-CoV-2 envelope (S2E) protein, the smallest of the major structural proteins, has shown that it can, by itself, exert significant deleterious effects.22 In vitro studies have demonstrated that the S2E protein can bind to human BBB-related cells, inhibit their viability in a dose- and time-dependent manner, and independently disrupt the barrier function of an in vitro BBB model. The S2E protein was also shown to trigger inflammatory responses in both brain endothelial cells and astrocytes.22 This indicates that even in the absence of active viral infection in the brain, the shedding of viral proteins into the circulation can be sufficient to initiate and sustain damage to the BBB, a mechanism that could be highly relevant in long COVID, where viral proteins may persist long after the acute phase.2
The Systemic Firestorm Breaches the Walls: Role of the Cytokine Storm
While direct viral effects can initiate damage, a major driver of BBB breakdown, particularly in severe acute COVID-19, is the profound systemic inflammatory response known as the "cytokine storm".7 This condition involves the massive and uncontrolled release of pro-inflammatory cytokines into the bloodstream. These circulating mediators, even if produced in distant organs like the lungs, act systemically and have a profound impact on the cerebral endothelium.
Key cytokines implicated in this process include interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β), all of which are found at significantly elevated levels in patients with moderate to severe COVID-19 and are particularly high in those with neurological symptoms like brain fog.3 These molecules are potent modulators of vascular permeability and act on brain endothelial cells through several distinct mechanisms to compromise BBB integrity:
Tight Junction Disassembly: The primary function of the BBB is to seal the paracellular pathway (the space between adjacent endothelial cells), a task accomplished by complex protein structures called tight junctions (e.g., claudins, occludin, and zonula occludens-1). Pro-inflammatory cytokines can trigger intracellular signaling cascades within endothelial cells that lead to the phosphorylation, internalization, and degradation of these key tight junction proteins. This dismantles the "molecular seal" between cells, creating a paracellular leak pathway.18
Cytoskeletal Reorganization: Cytokines can activate intracellular signaling pathways, such as the RhoA/ROCK pathway, which governs the cellular actin cytoskeleton.17 Activation of this pathway leads to the formation of stress fibers and acto-myosin contraction, which generates mechanical tension that physically pulls adjacent endothelial cells apart. This process of cytoskeletal reorganization directly contributes to the formation of intercellular gaps and a dramatic increase in paracellular permeability.17
Endothelial Activation and Immune Cell Adhesion: Cytokines act as powerful activators of the endothelium, shifting it from a quiescent, anti-inflammatory state to a pro-inflammatory, pro-thrombotic one. A key feature of this activation is the upregulation of cell adhesion molecules (CAMs) on the luminal surface of the endothelial cells, including Intercellular Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1).11 These molecules act as docking sites for circulating leukocytes (such as monocytes and T-cells), promoting their adhesion to the vessel wall. This adhesion is the critical first step in the process of immune cell transmigration across the BBB and into the brain parenchyma, seeding the CNS with peripheral inflammatory cells.
Immune-Mediated Vascular Injury: Friendly Fire on the Endothelium
Beyond the non-specific effects of the cytokine storm, the adaptive immune response to SARS-CoV-2 can itself become a source of targeted damage to the cerebral vasculature. This occurs through mechanisms that resemble an autoimmune attack on the components of the BBB. Autopsy studies of the brains of patients who died from COVID-19 have provided striking evidence for this process, revealing the widespread deposition of immune complexes (composed of IgG and IgM antibodies) and components of the complement system (C1q, C4d, and the terminal membrane attack complex, C5b-9) directly onto the surface of cerebral endothelial cells.23
The co-localization of antibodies and early complement components like C1q and C4d is the hallmark of classical complement pathway activation.23 This pathway is typically initiated when antibodies bind to their target antigens. In the context of COVID-19, it is hypothesized that antibodies generated against SARS-CoV-2 may cross-react with antigens present on the surface of endothelial cells, or alternatively, bind to viral antigens that have become lodged on the endothelial surface. Regardless of the precise target, the result is the formation of an immune complex that serves as a platform for complement activation. This activation unleashes a powerful inflammatory cascade that directly injures the endothelium through several mechanisms, including the generation of potent pro-inflammatory molecules and the assembly of the C5b-9 membrane attack complex, which can form pores in the cell membrane, leading to cell lysis and death.23 This antibody-mediated cytotoxicity directed against the endothelium is proposed to be a primary initiating event that leads to vascular leakage, platelet aggregation, and neuroinflammation.23
This immune-mediated pathology is not confined to the acute phase. Transcriptomic analyses of peripheral blood mononuclear cells (PBMCs) from individuals with long COVID reveal a persistent state of immune dysregulation, including a dampened adaptive immune response signature.6 In vitro experiments have shown that these PBMCs from long COVID patients exhibit significantly increased adhesion to human brain endothelial cells.6 This suggests a chronic state of immune activation where circulating immune cells are primed to interact with and potentially infiltrate the cerebral vasculature, perpetuating a cycle of low-grade inflammation and barrier dysfunction long after the initial infection has resolved.
Deconstruction of the Barrier: Enzymatic and Structural Failure
The final component of the molecular assault on the BBB involves the active deconstruction of its non-cellular structural elements, particularly the basement membrane. The BBB is not just a layer of cells; it is anchored to a specialized extracellular matrix known as the basement membrane, which provides critical structural support and contributes to the barrier's low permeability.
As previously introduced, both the virus and the subsequent inflammatory milieu trigger the upregulation and release of enzymes called matrix metalloproteinases (MMPs), with MMP-9 being of particular importance.14 MMP-9's primary substrate is type IV collagen, the main structural scaffold of the vascular basement membrane. By enzymatically cleaving and degrading this collagen, elevated MMP-9 activity effectively weakens the foundational support of the BBB, compromising its structural integrity and contributing significantly to its leakiness.17
This enzymatic degradation occurs in concert with the cellular damage to pericytes. As established, SARS-CoV-2 infection can lead to significant pericyte injury and loss.20 The combined effect of losing the cellular support from pericytes and the enzymatic degradation of the basement membrane by MMP-9 represents a comprehensive failure of the entire neurovascular unit's structural architecture. This multi-level damage explains why the resulting BBB disruption can be so profound and persistent. Restoring barrier function is therefore not simply a matter of reducing inflammation to allow endothelial cells to re-form their tight junctions; it likely requires a more complex process of vascular repair and regeneration to rebuild the damaged basement membrane and replenish the lost pericyte population. This comprehensive nature of the injury underscores the severity of the insult and the challenge in developing effective restorative therapies.
Table 2: Key Molecular Mechanisms of SARS-CoV-2-Induced Blood-Brain Barrier Disruption
Cerebral Microthrombosis: The Nexus of Thromboinflammation and Ischemic Injury
Parallel to the breakdown of the blood-brain barrier, a second, equally critical vascular pathology unfolds in the wake of SARS-CoV-2 infection: the formation of microscopic blood clots, or microthrombi, within the brain's smallest blood vessels. It is now widely understood that COVID-19 is not solely a respiratory disease but is also a profound vascular and thrombotic disorder.21 The systemic inflammation and endothelial injury triggered by the virus create a hypercoagulable environment that fosters the development of these micro-occlusions throughout the body, including the delicate cerebral microcirculation. This process, termed "thromboinflammation," represents a vicious cycle where inflammation drives thrombosis, and the resulting ischemia from thrombosis perpetuates further inflammation. This section will dissect the molecular pathways that lead to this pro-thrombotic state, explore the unique nature of the microclots found in long COVID, and detail the mechanisms by which these tiny occlusions inflict significant neuronal damage, ultimately contributing to the cognitive deficits characteristic of brain fog.
Pathways to a Pro-Thrombotic State: Endothelial Dysfunction and Hypercoagulability
The road to microthrombosis begins with the widespread endothelial dysfunction detailed in the previous section. A healthy endothelium maintains blood fluidity through a finely tuned balance of anticoagulant and procoagulant factors. SARS-CoV-2 infection catastrophically disrupts this balance, tipping the scales heavily in favor of clot formation.12
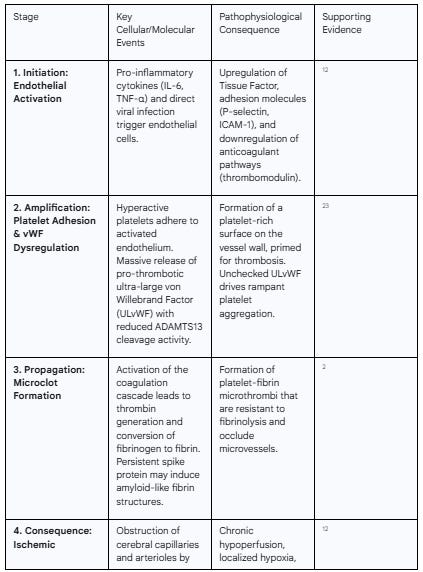
Endothelial Activation and Procoagulant Shift: As established, viral infection and the cytokine storm trigger a profound activation of endothelial cells. This activated state involves several key pro-thrombotic changes. Firstly, endothelial cells begin to express Tissue Factor on their surface, the primary initiator of the extrinsic coagulation cascade, which leads to the generation of thrombin and the formation of fibrin clots.12 Secondly, they upregulate the expression of adhesion molecules such as
P-selectin, ICAM-1, and VCAM-1. These molecules not only facilitate leukocyte adhesion but also act as docking sites for platelets, promoting their aggregation at the site of vascular injury.12 Thirdly, the activated endothelium downregulates its natural anticoagulant systems. The expression of molecules like thrombomodulin and the endothelial protein C receptor, which are crucial for activating the protein C pathway that inhibits coagulation, is reduced.12 This simultaneous upregulation of pro-coagulant factors and downregulation of anticoagulant factors creates a potent pro-thrombotic surface along the vessel wall.Platelet Hyperactivation: Platelets, the key cellular mediators of thrombosis, become hyperactive in response to SARS-CoV-2 infection and the associated inflammation. They exhibit increased surface expression of activation markers and a greater propensity to aggregate.26 Autopsy studies of COVID-19 brains have confirmed the presence of widespread platelet aggregates and microthrombi, often found adhering directly to the activated endothelial cells lining the vascular lumen.23 This platelet hyperactivation is a central driver of microthrombus formation.
The Dysregulated von Willebrand Factor (vWF)-ADAMTS13 Axis: A critical molecular pathway underpinning COVID-19 coagulopathy is the severe derangement of the vWF-ADAMTS13 axis.25 Von Willebrand Factor is a large multimeric protein stored within endothelial cells. Upon endothelial injury or activation, it is released in the form of ultra-large vWF (ULvWF) multimers. These ULvWF strands are exceptionally pro-thrombotic, rapidly binding to platelets and tethering them to the vessel wall, especially under conditions of high shear stress found in the microcirculation.25 In a healthy state, the pro-thrombotic activity of ULvWF is tightly controlled by a specific cleaving protease called ADAMTS13, which snips the ultra-large multimers into smaller, less active forms. In severe COVID-19, this regulatory system breaks down. Endothelial injury leads to a massive release of ULvWF, while at the same time, levels and activity of ADAMTS13 are significantly reduced.25 This creates a profound imbalance, leaving the hyperactive ULvWF multimers unchecked to drive rampant platelet aggregation and microthrombosis.25
The Pathophysiology of Persistent Fibrin Amyloid Microclots
While the mechanisms above explain the hypercoagulability seen in acute COVID-19, a particularly unique and potentially crucial feature has been identified in the context of long COVID: the presence of persistent, anomalous microclots in the circulation.2 These are not typical fibrin clots. Through specialized fluorescence microscopy techniques, researchers have discovered that the plasma of many long COVID patients contains significant quantities of fibrin-rich microclots that have adopted an "amyloid" conformation. This altered protein structure makes them highly resistant to the body's natural process of fibrinolysis, the enzymatic breakdown of clots.28
The persistence of these fibrinolysis-resistant microclots is proposed to be a central driver of long COVID symptoms.2 By continuously circulating and lodging in the body's smallest capillaries, they can cause widespread microvascular occlusion, leading to tissue hypoxia.29 The trigger for the formation of these abnormal clots is thought to be the persistence of SARS-CoV-2 spike protein in the circulation for months after the acute infection has resolved.2 The spike protein has been shown to be capable of directly inducing conformational changes in fibrinogen, causing it to polymerize into this pathological amyloid form. Therefore, the lingering presence of viral components may be the direct cause of this ongoing thromboinflammatory state, continuously generating microclots that the body cannot effectively clear.2
This hypothesis is supported by biochemical evidence. Many individuals with long COVID exhibit persistently elevated levels of D-dimer, a degradation product of fibrin that indicates ongoing clot formation and breakdown, for months after their initial infection.12 This sustained coagulation activation, coupled with the discovery of these unique, degradation-resistant microclots, provides a powerful explanation for the chronic and relapsing nature of many long COVID symptoms, including brain fog.
Mechanisms of Microvascular Occlusion-Induced Neuronal Damage
The cumulative burden of countless microthrombi lodging in the cerebral microcirculation, though often too small to be detected by conventional neuroimaging, inflicts substantial damage on the surrounding neural tissue through several interrelated mechanisms.12 The primary consequence of these micro-occlusions is a profound disruption of local blood supply.
Chronic Hypoperfusion and Localized Hypoxia: Each microthrombus acts as a dam, obstructing or severely reducing blood flow in the downstream capillary network. This leads to a state of chronic hypoperfusion, where the affected brain tissue does not receive an adequate supply of oxygen and glucose.12 This creates pockets of localized hypoxia (low oxygen) and metabolic stress. Brain regions with high metabolic activity, such as the hippocampus (critical for memory) and the prefrontal cortex (essential for executive function), are particularly vulnerable to even subtle reductions in perfusion.12 The widespread, multifocal nature of this hypoperfusion can disrupt the function of large-scale neural networks that underpin higher-order cognition.
Mitochondrial Dysfunction and Neuronal Apoptosis: Neurons have extremely high energy demands, which are met by mitochondria through aerobic respiration. When oxygen supply is compromised by hypoperfusion, mitochondria can no longer function efficiently, leading to a state of energy failure. This metabolic crisis triggers intracellular stress pathways that can culminate in neuronal apoptosis, or programmed cell death.12 Histological analyses of brain tissue from long COVID patients have provided evidence of sustained ischemic injury, including apoptotic neuronal loss and mitochondrial fragmentation, confirming that this process occurs in vivo.12
Impaired Synaptic Homeostasis: Normal synaptic transmission and plasticity, the cellular basis of learning and memory, are exquisitely sensitive to the local metabolic environment. The chronic hypoperfusion caused by microthrombosis disrupts the delicate homeostasis required for these processes. The reduced availability of ATP impairs the function of ion pumps needed to maintain neuronal resting potentials, the synthesis and recycling of neurotransmitters, and the structural maintenance of synapses.12 This impairment of synaptic homeostasis is a direct cellular mechanism that can manifest clinically as the core symptoms of brain fog: slowed processing speed, difficulty with memory recall, and reduced attention span.13
Autopsy and Histological Evidence of Widespread Microvascular Injury
The most definitive confirmation of this thromboinflammatory pathology comes from post-mortem histological examination of brain tissue from patients who died from COVID-19. These studies provide a direct microscopic view of the consequences of the disease on the cerebral vasculature.
Autopsy findings consistently reveal evidence of widespread microvascular injury. This includes congested blood vessels, leakage of blood proteins like fibrinogen into the brain parenchyma, and, most importantly, the clear presence of platelet-rich microthrombi and platelet aggregates adhering to the endothelial lining of small vessels.20 These pathological findings are not confined to a single brain region but are often multifocal, supporting the idea of a systemic process affecting the entire brain. Furthermore, the presence of cerebral microbleeds, which are small hemorrhages resulting from the rupture of damaged microvessels, is also a common finding and is strongly correlated with the severity of the illness and increased mortality rates.30 This direct histological evidence validates the mechanisms inferred from circulating biomarkers and in vitro studies, confirming that microthrombosis is a real and significant feature of the neurovascular pathology of COVID-19.
Table 3: The Thromboinflammatory Cascade in the Cerebral Microvasculature
Synthesizing the Pathology: The Mechanistic Link Between Neurovascular Injury and Cognitive Deficits in Long COVID
The preceding sections have established two parallel, yet interconnected, streams of neurovascular pathology in long COVID: a persistent disruption of the blood-brain barrier leading to a neuroinflammatory state, and a sustained thromboinflammatory process resulting in widespread cerebral microthrombosis and ischemia. While each of these pathologies is damaging in its own right, their true impact on cognitive function arises from their synergistic interplay. This final analytical section will synthesize the evidence to construct a unified model that explains how the combination of a "leaky" and "clogged" cerebral microvasculature gives rise to the debilitating cognitive deficits, particularly brain fog, that characterize neurological long COVID. The clinical symptoms are not the result of a single insult but are the emergent property of a complex, self-perpetuating cycle of neuroinflammation and metabolic failure.
From Leaky Vessels to Impaired Cognition: Neuroinflammation and Disrupted Homeostasis
The primary consequence of a chronically compromised BBB is the loss of the brain's immune privilege and the disruption of its meticulously controlled homeostatic environment. An intact BBB normally prevents the entry of peripheral immune cells and potentially neurotoxic molecules from the bloodstream into the delicate brain parenchyma. When this barrier becomes permeable, as demonstrated by the imaging and biomarker evidence in long COVID, it opens the floodgates for a host of pathological agents.12
This influx includes circulating pro-inflammatory cytokines, autoantibodies, and blood-derived proteins such as fibrinogen and thrombin, which are themselves inflammatory when present in the brain tissue.23 Furthermore, the leaky barrier facilitates the infiltration of peripheral immune cells, including monocytes (which can differentiate into macrophages) and T-cells, which are drawn to the site of vascular injury.23 This sustained entry of inflammatory elements from the periphery triggers a state of chronic neuroinflammation. This is characterized by the activation of the brain's resident immune cells: microglia and astrocytes.12
While acute activation of these glial cells is a protective response, their chronic activation becomes neurotoxic. Activated microglia and astrocytes release a barrage of their own inflammatory mediators, including reactive oxygen species (ROS), nitric oxide, and additional cytokines, creating a self-amplifying inflammatory cycle within the brain parenchyma.12 This neuroinflammatory milieu is profoundly disruptive to normal neuronal function. It directly impairs synaptic plasticity—the ability of synapses to strengthen or weaken over time, which is the cellular basis of learning and memory. It can lead to "synaptic stripping" by overactive microglia, disrupting established neural circuits. Ultimately, if the inflammation is severe and prolonged, it can induce neuronal death.16 This inflammation-driven disruption of synaptic signaling and network integrity is a direct biological cause of cognitive impairment, manifesting as the memory lapses, slowed thought processes, and difficulty concentrating reported by patients with brain fog.
The Cumulative Burden of Micro-Ischemia: "Energy Failure" in the Brain
Concurrently with the inflammatory insult, the widespread microthrombosis described in Section 3 inflicts a different, yet equally damaging, form of injury: chronic metabolic stress. The cumulative burden of countless micro-occlusions throughout the cerebral vasculature leads to a state of chronic hypoperfusion, creating a mosaic of tiny ischemic zones across the brain.12
This pathology can be conceptualized as an "energy failure" in the brain. Neurons, particularly those involved in higher-order cognitive functions located in the prefrontal and temporal cortices, are among the most metabolically demanding cells in the body. They require a constant and substantial supply of oxygen and glucose to fuel the immense energy expenditure needed for maintaining ion gradients, synthesizing neurotransmitters, and supporting synaptic activity.12
Even subtle, chronic reductions in local blood flow can push these high-demand neurons to the brink of metabolic collapse. The resulting hypoxia impairs mitochondrial function, drastically reducing ATP production. Without sufficient energy, neurons cannot fire properly, synaptic transmission becomes inefficient, and the complex, coordinated activity of large-scale neural networks required for tasks like working memory, attentional focus, and rapid information processing begins to fail. This ischemia-driven metabolic failure provides a powerful and direct explanation for many of the classic symptoms of brain fog. The feeling of mental slowness or "fuzziness" can be directly attributed to the brain's processing power being throttled by an inadequate energy supply.1
A Unified Model of Neurovascular Injury in Long COVID
The most accurate model for understanding the neurological consequences of long COVID is one that recognizes the synergistic and mutually reinforcing nature of BBB disruption and microthrombosis. These are not independent events but two facets of a single, overarching thromboinflammatory process that creates a devastating "two-hit" assault on the brain.
"Hit 1" is the leaky BBB. This breach creates a toxic, inflammatory brain environment. It allows inflammatory cells and molecules to enter the CNS, "poisoning" the neural microenvironment and directly disrupting synaptic function.
"Hit 2" is the microthrombosis. This process creates widespread hypoperfusion, "starving" neurons of the oxygen and glucose they need to function and to cope with the inflammatory stress imposed by the first hit.
These two processes feed into each other in a destructive positive feedback loop. The inflammatory environment created by the leaky BBB (Hit 1) further activates the endothelium and platelets, exacerbating the pro-thrombotic state and promoting more microthrombosis (Hit 2).12 In turn, the ischemic conditions and hypoxia created by microthrombosis (Hit 2) cause further damage to the endothelial cells, increasing the permeability of the BBB (Hit 1) and perpetuating the cycle of inflammation.
This unified model, where inflammation-driven synaptic disruption and ischemia-driven metabolic failure occur simultaneously and amplify one another, provides a robust and comprehensive explanation for the severity, persistence, and multifaceted nature of the cognitive deficits seen in long COVID. It moves beyond a simplistic, single-cause explanation and embraces the complexity of the neurovascular injury, thereby providing a more solid foundation for the development of targeted, multi-modal therapeutic strategies.
Diagnostic and Therapeutic Horizons
The detailed mechanistic understanding of BBB disruption and microthrombosis as central drivers of neurological long COVID is not merely an academic exercise; it has profound implications for clinical practice. It provides a clear rationale for the development of novel diagnostic tools capable of objectively measuring this neurovascular injury and points directly toward targeted therapeutic strategies aimed at restoring vascular integrity and resolving the underlying thromboinflammation. The future of managing long COVID brain fog lies in moving from symptom management to a precision-medicine approach guided by a biological understanding of the disease.
Advanced Diagnostic Modalities for Quantifying Neurovascular Injury
The diagnosis of neurological long COVID currently relies heavily on patient-reported symptoms, which, while valid, lack objectivity. The pathologies described in this report are, however, measurable. The deployment of advanced diagnostic modalities in clinical and research settings is crucial for objectively identifying patients with neurovascular injury, quantifying its severity, monitoring disease progression, and assessing the response to treatment.
Magnetic Resonance Imaging (MRI): While standard structural MRI is often unrevealing, specialized MRI sequences are proving invaluable.
Permeability Imaging: Techniques like DCE-MRI and non-contrast methods such as WEPCAST and diffusion-weighted arterial spin labeling (DW-ASL) should be considered key tools for quantifying BBB leakage. Their ability to generate regional maps of permeability can help correlate the location of vascular damage with specific symptom profiles.4
Thrombosis and Microbleed Imaging: High-resolution sequences such as T2-weighted gradient-echo (T2-GRE)** and susceptibility-weighted imaging (SWI) are highly sensitive to the magnetic susceptibility effects of blood products. These sequences can detect the presence of cerebral microbleeds and may have the potential to visualize intravascular microthrombi as hypointense vessels, providing a non-invasive window into the thrombotic burden.35
Perfusion Imaging: Arterial spin labeling (ASL) can non-invasively map cerebral blood flow, allowing for the detection of the hypoperfusion that results from microvascular occlusions.
Positron Emission Tomography (PET): PET imaging offers the unique ability to visualize specific molecular processes in vivo.
Neuroinflammation Imaging: The development of PET radioligands that bind to the 18 kDa translocator protein (TSPO), which is upregulated in activated microglia and astrocytes, allows for the direct imaging of neuroinflammation. Tracers such as [11C]PBR28 and [18F]DPA-714 have already been used to demonstrate widespread neuroinflammation in long COVID patients.1 Combining PET with MRI (PET-MRI) can co-register this inflammatory activity with anatomical and permeability data, providing a comprehensive picture of the neurovascular pathology.
Blood-Based Biomarkers: A panel of carefully selected blood biomarkers could provide a more accessible, scalable, and cost-effective method for screening and monitoring patients. Based on the established pathophysiology, a promising panel would include markers of BBB breach (S100β), basement membrane degradation (MMP-9), astrocyte injury (GFAP), neuronal damage (NFL), and ongoing coagulation (D-dimer).5 Serial monitoring of these markers could track disease activity and response to therapy.
Emerging Therapeutic Strategies: From Barrier Repair to Thrombus Resolution
A clear understanding of the disease mechanism illuminates a path toward rational, targeted therapies. Instead of treating symptoms, future interventions can aim to correct the underlying neurovascular pathology.
Targeting Blood-Brain Barrier Repair: A novel and exciting therapeutic avenue is the development of drugs that directly stabilize and repair the leaky BBB.
Wnt/β-catenin Pathway Agonists: The Wnt/β-catenin signaling pathway is known to be critical for the development and maintenance of BBB integrity. Preclinical research in mouse models of COVID-19 has shown that the infection suppresses this pathway, leading to a leaky BBB and cognitive deficits. Encouragingly, treatment with engineered ligands that specifically activate this pathway (e.g., engineered Wnt7a ligands or molecules targeting the FZD4 receptor) has been shown to reduce BBB leakage, decrease immune cell infiltration into the brain, and rescue cognitive impairments in these models.40 This represents a promising strategy for directly "sealing" the barrier.
Regenerative Therapies: More experimental approaches, such as the use of stem cells and their derived exosomes, are being explored for their potential to promote vascular repair and regeneration. These therapies may work through anti-inflammatory, antioxidant, and pro-angiogenic mechanisms to help rebuild the damaged neurovascular unit.43
Resolving Thromboinflammation: Addressing the persistent microclots is another key therapeutic goal.
Targeted Antithrombotic Therapy: The inconsistent results from trials of standard anticoagulants suggest that the unique, fibrinolysis-resistant amyloid microclots in long COVID may require a different approach.2 Preliminary, non-randomized studies investigating an aggressive "triple therapy" regimen—typically combining two antiplatelet agents (e.g., aspirin and clopidogrel) with a direct oral anticoagulant (e.g., apixaban)—have reported promising results, with many patients experiencing significant symptom improvement and a reduction in detectable microclots.28 However, this approach carries a significant risk of bleeding and remains highly experimental. Its efficacy and safety must be rigorously evaluated in large-scale, randomized controlled trials before any clinical recommendations can be made.
Complementary Strategies:
Anti-inflammatory and Immunomodulatory Agents: Given the central role of inflammation, agents such as corticosteroids, mast cell stabilizers, and antihistamines may play a role in dampening the systemic and central inflammatory responses.1
Antiviral Therapy: If persistent viral reservoirs are indeed a primary driver of ongoing thromboinflammation and endothelial dysfunction, then a course of antiviral therapy (e.g., nirmatrelvir/ritonavir) long after the acute phase could theoretically help to eliminate the root cause of the problem.15 The efficacy of this strategy is currently under investigation.
The diverse nature of the underlying pathology strongly suggests that a "one-size-fits-all" approach to treating neurological long COVID is unlikely to succeed. The future of effective clinical management will likely depend on a personalized or stratified approach. Using the advanced diagnostic tools described above, clinicians may one day be able to profile an individual patient's specific neurovascular phenotype. A patient with evidence of high BBB permeability and neuroinflammation (e.g., high S100β, positive PET scan) but normal coagulation markers might be a candidate for a barrier-stabilizing therapy. Conversely, a patient with highly elevated D-dimer, evidence of microclots, and perfusion deficits on MRI might benefit more from a targeted antithrombotic regimen. This diagnostic-guided stratification will be essential for designing successful clinical trials and ultimately for delivering the right treatment to the right patient.
Conclusion: Recalibrating Our Understanding of Long COVID as a Primary Neurovascular Disease
The extensive body of evidence synthesized in this report converges on an unequivocal conclusion: the neurological manifestations of long COVID, particularly the debilitating syndrome of "brain fog," are not an enigmatic post-viral fatigue state but are the direct and measurable consequences of a sustained and complex neurovascular pathology. The data from advanced neuroimaging, circulating biomarkers, and post-mortem histology paint a coherent picture of a disease process centered on the brain's microvasculature.
The central thesis of this report is that a synergistic, self-perpetuating interplay between two primary pathological processes—a persistently leaky blood-brain barrier and chronic cerebral microthrombosis—provides a robust, evidence-based framework for understanding the cognitive deficits that afflict millions of individuals worldwide. The leaky BBB fosters a chronic neuroinflammatory state, disrupting synaptic function and neuronal homeostasis, while widespread microthrombosis leads to chronic hypoperfusion and metabolic failure in high-demand cognitive centers. Together, these insults create a cerebral environment that is both "poisoned" by inflammation and "starved" of energy, fully accounting for the profound impairments in memory, attention, and executive function observed clinically.
This recalibration of long COVID as a primary neurovascular disease has critical implications. It validates the experiences of patients by grounding their symptoms in objective, measurable pathophysiology. It provides a clear roadmap for research, focusing efforts on the molecular mechanisms of endothelial injury, immune dysregulation, and persistent coagulopathy. Most importantly, it illuminates a new landscape of rational diagnostic and therapeutic strategies. By targeting the fundamental drivers of the disease—the compromised barrier and the occlusive microclots—we can move beyond mere symptom management and toward the development of truly disease-modifying therapies aimed at restoring neurovascular health and, in doing so, giving patients back their cognitive clarity and quality of life. The path forward requires a concerted effort to translate these scientific insights into clinical tools and treatments, transforming our understanding of long COVID into tangible hope for those affected.
Acknowledgement
I acknowledge the use of Gemini AI in the preparation of this report. Specifically, it was used to: (1) brainstorm and refine the initial research questions; (2) assist in writing and debugging Python scripts for statistical analysis; and (3) help draft, paraphrase, and proofread sections of the final manuscript. I reviewed, edited, and assume full responsibility for all content.
Works cited
Neurological sequelae of long COVID: a comprehensive ... - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2024.1465787/full
What Role Does Microthrombosis Play in Long COVID? | Request PDF, accessed August 31, 2025, https://www.researchgate.net/publication/374174602_What_Role_Does_Microthrombosis_Play_in_Long_COVID
Disruption of the blood-brain barrier due to long COVID - News-Medical, accessed August 31, 2025, https://www.news-medical.net/news/20230127/Disruption-of-the-blood-brain-barrier-due-to-long-COVID.aspx
Blood–brain barrier disruption and sustained systemic inflammation ..., accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10917679/
Long Covid 'brain fog' may be due to leaky blood-brain barrier, study finds - The Guardian, accessed August 31, 2025, https://www.theguardian.com/society/2024/feb/22/long-covid-brain-fog-may-be-due-to-leaky-blood-brain-barrier-study
Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment - PubMed, accessed August 31, 2025, https://pubmed.ncbi.nlm.nih.gov/38388736/
(PDF) Blood–brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment - ResearchGate, accessed August 31, 2025, https://www.researchgate.net/publication/378400466_Blood-brain_barrier_disruption_and_sustained_systemic_inflammation_in_individuals_with_long_COVID-associated_cognitive_impairment
Science Direct: Blood-Brain barrier disruption in long COVID and ..., accessed August 31, 2025, https://meassociation.org.uk/2025/07/science-direct-blood-brain-barrier-disruption-in-long-covid-and-cognitive-correlates/
Blood-Brain barrier disruption in long COVID and cognitive correlates: A cross-sectional MRI study - PubMed, accessed August 31, 2025, https://pubmed.ncbi.nlm.nih.gov/40738266/
Blood-Brain barrier disruption in long COVID and cognitive correlates: A cross-sectional MRI study - Johns Hopkins University, accessed August 31, 2025, https://pure.johnshopkins.edu/en/publications/blood-brain-barrier-disruption-in-long-covid-and-cognitive-correl
Blood-brain barrier disruption in Long COVID-associated cognitive impairment, accessed August 31, 2025, https://www.researchgate.net/publication/367354794_Blood-brain_barrier_disruption_in_Long_COVID-associated_cognitive_impairment
The Molecular Mechanisms of Cognitive Dysfunction in Long COVID ..., accessed August 31, 2025, https://www.mdpi.com/1422-0067/26/11/5102
The Molecular Mechanisms of Cognitive Dysfunction in Long COVID ..., accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12154490/
Markers of blood-brain barrier disruption increase early and ..., accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9798841/
Cerebromicrovascular mechanisms contributing to long COVID: implications for neurocognitive health - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11872997
Vascular Dysfunctions Contribute to the Long-Term Cognitive Deficits Following COVID-19, accessed August 31, 2025, https://www.mdpi.com/2079-7737/12/8/1106
Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10043238/
(PDF) A review of the mechanisms of blood–brain barrier disruption during COVID-19 infection - ResearchGate, accessed August 31, 2025, https://www.researchgate.net/publication/372439496_A_review_of_the_mechanisms_of_blood-brain_barrier_disruption_during_COVID-19_infection
COVID-19: Cellular and Molecular Mechanisms of Brain Damage ..., accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8985060/
SARS-CoV-2 induces blood-brain barrier and choroid plexus barrier impairments and vascular inflammation in mice - PMC - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11446308/
[Translated article] Pulmonary Vascular Tone Dysregulation and Microthrombosis in COVID-19 | Archivos de Bronconeumología, accessed August 31, 2025, https://www.archbronconeumol.org/en-translated-article-pulmonary-vascular-tone-articulo-S0300289622000758
The SARS-CoV-2 envelope protein disrupts barrier function in an in vitro human blood-brain barrier model - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2022.897564/full
Neurovascular injury with complement activation and inflammation ..., accessed August 31, 2025, https://academic.oup.com/brain/article/145/7/2555/6621999
The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications, accessed August 31, 2025, https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.120.317447
COVID-19 and Delayed Cerebral Ischemia—More in Common Than ..., accessed August 31, 2025, https://www.mdpi.com/2077-0383/10/12/2646
Antiplatelet therapy for patients with COVID-19: Systematic review and meta-analysis of observational studies and randomized controlled trials - PMC, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9490267/
Thromboinflammation in long COVID—the elusive key to ..., accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10174338/
Long COVID: Could treating microclots relieve symptoms? - MedicalNewsToday, accessed August 31, 2025, https://www.medicalnewstoday.com/articles/long-covid-could-antiplatelet-therapy-help
A Blood Supply Pathophysiological Microcirculatory Mechanism for ..., accessed August 31, 2025, https://www.mdpi.com/2075-1729/14/9/1076
Cerebral microbleeds in patients with COVID-19: is there an inevitable connection? | Brain Communications | Oxford Academic, accessed August 31, 2025, https://academic.oup.com/braincomms/article/6/5/fcae236/7717184
A Comprehensive View on MRI Techniques for Imaging Blood-Brain Barrier Integrity - Maastricht University, accessed August 31, 2025, https://cris.maastrichtuniversity.nl/en/publications/a-comprehensive-view-on-mri-techniques-for-imaging-blood-brain-ba
Magnetic resonance imaging of blood–brain barrier permeability in ischemic stroke using diffusion- weighted arterial spin labe - Renaissance School of Medicine, accessed August 31, 2025, https://renaissance.stonybrookmedicine.edu/sites/default/files/temp1_0.pdf
A Comprehensive View on MRI Techniques for Imaging Blood-Brain Barrier Integrity - Maastricht University, accessed August 31, 2025, https://cris.maastrichtuniversity.nl/files/75664118/Jansen_2020_A_Comprehensive_View_on_MRI.pdf
In vivo methods for imaging blood–brain barrier function and dysfunction - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9931809/
Ultra-Early Cerebral Thrombosis Formation After Experimental Subarachnoid Hemorrhage Detected on T2* Magnetic Resonance Imaging | Stroke - American Heart Association Journals, accessed August 31, 2025, https://www.ahajournals.org/doi/10.1161/STROKEAHA.120.032397
Neuroinflammation and "brain fog" in Long COVID & ME/CFS, accessed August 31, 2025, https://polybio.org/projects/5272/
Long COVID is associated with extensive in-vivo neuroinflammation on [ 18 F]DPA-714 PET, accessed August 31, 2025, https://www.medrxiv.org/content/10.1101/2022.06.02.22275916v1
Long COVID is associated with extensive in-vivo neuroinflammation on [18F]DPA-714 PET, accessed August 31, 2025, https://www.medrxiv.org/content/10.1101/2022.06.02.22275916v1.full-text
Neuroinflammation in post-acute sequelae of COVID-19 (PASC) as assessed by [11C]PBR28 PET correlates with vascular disease measures - PubMed Central, accessed August 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10614970/
New Therapeutic Target for Clearing post-Covid Brain Fog - Global Autoimmune Institute, accessed August 31, 2025, https://www.autoimmuneinstitute.org/covid_timeline/new-therapeutic-target-for-clearing-post-covid-brain-fog/
Research offers hope for preventing post-COVID 'brain fog' by targeting brain's blood vessels - UIC today - University of Illinois Chicago, accessed August 31, 2025, https://today.uic.edu/covid-brain-fog-research/
Restoring the blood-brain barrier? - Stanford Medicine, accessed August 31, 2025, https://med.stanford.edu/news/insights/2023/06/restoring-the-blood-brain-barrier.html
Blood-brain barrier repair: potential and challenges of stem cells and exosomes in stroke treatment - Frontiers, accessed August 31, 2025, https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2025.1536028/full
Triple Anticoagulant Therapy for Long COVID: A Comprehensive Overview - Pain Spa, accessed August 31, 2025, https://www.painspa.co.uk/triple-anticoagulant-therapy-for-long-covid-a-comprehensive-overview/