Defining Post-COVID Immune Dysfunction: A Multi-Modal Biomarker Framework for Diagnosis, Stratification, and Personalized Therapy
Long COVID stems from persistent immune dysfunction involving viral remnants, autoimmunity, latent virus reactivation, and vascular damage—requiring personalized biomarker-based diagnosis.
The Immunological Pathophysiology of Post-Acute Sequelae of SARS-CoV-2 (PASC)
Post-Acute Sequelae of SARS-CoV-2 (PASC), colloquially known as Long COVID, represents a complex and debilitating condition affecting a significant proportion of individuals following acute infection.1 It is increasingly understood not as a simple persistence of symptoms, but as a multifaceted syndrome driven by profound and sustained immune dysregulation.3 The condition is marked by the continuation of initial symptoms or the emergence of new ones four or more weeks after the acute phase, encompassing over 200 symptoms that can affect nearly every organ system.2 This clinical heterogeneity is a cardinal feature and a primary challenge, suggesting that the underlying immunopathology is not monolithic but varies significantly based on the patient's clinical phenotype, the severity of their initial COVID-19 illness, and the specific tissues involved.4 The development of a robust diagnostic and therapeutic framework for PASC is therefore contingent on deciphering this complex immunological landscape.
The Persistent State of Immune Dysregulation: A Failure to Reset Homeostasis
A central theme emerging from extensive research is that PASC is characterized by an immune system that fails to return to a state of homeostasis following the clearance of acute viral infection. Instead, it remains in a state of "constant high alert".8 This chronic dysregulation involves pathological alterations in both the innate and adaptive branches of the immune system.4 Unlike the exuberant but typically self-limiting immune response seen in acute COVID-19, the immunopathology of PASC appears to be a distinct, chronic process.4 This persistent immune activation is considered a key pathogenic feature driving the systemic and tissue-specific manifestations of the disease.1
The condition's remarkable heterogeneity, with symptoms ranging from debilitating fatigue and post-exertional malaise (PEM) to autonomic dysfunction, cognitive impairment ("brain fog"), and cardiovascular complications, strongly implies that a single pathogenic mechanism is unlikely to account for all clinical presentations.2 This variability necessitates a move away from a one-size-fits-all diagnostic approach toward one that can stratify patients into more homogeneous subgroups based on underlying biological drivers. Further complicating the picture are the mechanistic parallels between PASC and other post-viral syndromes, most notably Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), which also feature symptoms of PEM, cognitive dysfunction, and immune alterations, suggesting that SARS-CoV-2 may trigger common, final pathways of post-infectious pathology.3
The Four Mechanistic Pillars of PASC Immunopathology
Current evidence points toward four primary, interconnected hypotheses that collectively explain the sustained immune dysregulation observed in PASC. These pillars—viral persistence, autoimmunity, latent virus reactivation, and endothelial dysfunction—are not mutually exclusive but likely interact to create a self-perpetuating cycle of inflammation and tissue damage.
Pillar 1: Viral Persistence
A compelling body of evidence suggests that SARS-CoV-2 or its components can persist in the body long after the acute infection has resolved. The virus may establish reservoirs in immunologically privileged or less-sampled tissues, such as the gastrointestinal tract, central nervous system, olfactory system, and endothelium, thereby evading systemic immune clearance.3 Studies have detected viral RNA and proteins in various extrapulmonary tissues for weeks or even months post-infection, even in cases where viral RNA is undetectable in the plasma.11 These viral reservoirs are hypothesized to act as a source of persistent antigen, continually stimulating local and systemic immune responses.3 This chronic antigenic stimulation drives cytokine imbalances, T-cell exhaustion, and systemic inflammation, contributing directly to the persistent symptoms of PASC.3 Furthermore, extracellular vesicles (EVs) have been implicated as potential viral reservoirs, capable of transporting viral fragments through the circulatory system, thereby disseminating inflammatory triggers to distant sites.11
Pillar 2: Autoimmunity
The dysregulated immune response during and after acute COVID-19 can lead to a break in self-tolerance, resulting in the production of a wide array of autoantibodies.1 The failure to clear these autoantibodies following the acute phase is a leading hypothesis for the pathogenesis of PASC.9 Multiple studies have identified various autoantibodies in PASC patients, including those targeting nuclear antigens (ANAs), G-protein coupled receptors (GPCRs), and components of the autonomic nervous system, which may underlie symptoms like orthostatic intolerance and POTS.9
Crucially, the role of autoantibodies has been elevated from mere association to a potential causal factor through elegant antibody transfer studies in animal models. Research led by Akiko Iwasaki demonstrated that when purified immunoglobulin G (IgG) from PASC patients was transferred into healthy mice, the animals began to exhibit PASC-like symptoms, including heightened pain sensitivity, dizziness, and muscle weakness.15 The specificity of this effect was remarkable: antibodies from patients reporting pain were most likely to induce pain sensitivity in mice, while antibodies from patients reporting dizziness were most likely to induce balance deficits.15 This provides powerful evidence that in a substantial subset of patients, specific autoantibodies are directly pathogenic, targeting self-antigens in various tissues to produce distinct clinical symptoms.
Pillar 3: Reactivation of Latent Viruses
The state of chronic immune dysregulation and T-cell exhaustion characteristic of PASC creates a permissive environment for the reactivation of latent herpesviruses that are typically held in check by a healthy immune system.2 Researchers have documented evidence of the reactivation of viruses such as Epstein-Barr virus (EBV), human herpesvirus 6 (HHV-6), and cytomegalovirus (CMV) in individuals with PASC.8 The reactivation of these viruses introduces an additional layer of antigenic stimulation and inflammation, which can further tax the already dysregulated immune system and contribute to the overall symptom burden, potentially confounding the specific immune response to SARS-CoV-2 antigens.
Pillar 4: Endothelial Dysfunction and Coagulopathy
The vascular endothelium is a key target of both direct viral infection by SARS-CoV-2 and indirect damage from chronic inflammation.3 Persistent viral presence in endothelial cells, coupled with the ongoing inflammatory milieu, leads to a state of profound endothelial dysfunction.11 This is characterized by the increased expression of cell adhesion molecules such as ICAM-1 and VCAM-1, which facilitates leukocyte adhesion and infiltration into tissues.11 Concurrently, this process promotes platelet hyperactivation and disrupts vascular homeostasis, leading to a prothrombotic state.11 A particularly notable finding is the formation of amyloid-containing, fibrinoid microclots in the plasma of PASC patients, which are resistant to normal fibrinolysis and may contribute to microvascular occlusion, tissue hypoxia, and multi-organ symptoms.11 This pillar directly links the immune system's dysfunction to the widespread vascular and coagulation abnormalities observed in the condition.12
The four mechanistic pillars of PASC do not operate in isolation but rather form a complex, self-sustaining pathological feedback loop. The persistence of viral antigens (Pillar 1) serves as the foundational and ongoing trigger for the immune system. This chronic stimulation drives relentless T- and B-cell activation, which can breach central and peripheral tolerance mechanisms, leading to the generation of pathogenic autoantibodies against self-tissues (Pillar 2). The resulting systemic immune dysregulation, characterized by features like T-cell exhaustion, creates an environment where control over latent pathogens is lost, allowing for their reactivation (Pillar 3), which adds a new source of inflammation and antigenic burden. The combined inflammatory assault from these three sources inflicts direct damage on the vascular endothelium (Pillar 4), causing microvascular dysfunction, coagulopathy, and tissue hypoxia. This vascular pathology may, in turn, create protected niches that shield viral reservoirs from immune surveillance, thus reinforcing the initial trigger and completing the vicious cycle. This interconnected pathophysiology underscores the absolute necessity for a multi-modal biomarker panel. A diagnostic approach focused on a single marker, such as a cytokine, would be insufficient as it would only capture one facet of this intricate system. A robust panel must be capable of probing each component of this feedback loop to provide a comprehensive picture of an individual patient's disease.
Candidate Biomarkers: Cytokine and Chemokine Signatures
The persistent state of immune activation in PASC is reflected in the circulation of soluble immune mediators, including cytokines and chemokines. Analysis of these molecules offers a window into the specific inflammatory pathways that are active in patients. However, the narrative is more complex than the simple "cytokine storm" described in acute, severe COVID-19, with evidence pointing toward distinct and sometimes contradictory inflammatory signatures that may define different patient subgroups.
The Chronic Pro-Inflammatory Milieu
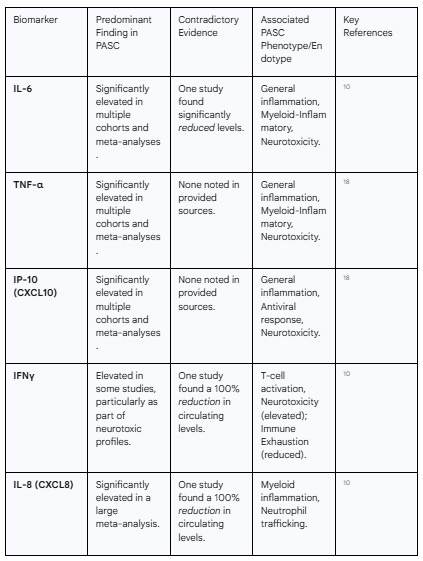
A consistent finding across numerous studies is that PASC is associated with a state of chronic, low-grade inflammation. A comprehensive meta-analysis encompassing 82 studies and over 3,800 PASC patients confirmed a significant elevation of multiple pro-inflammatory cytokines and chemokines compared to healthy controls.18 Among the most consistently elevated markers are Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α), and Interferon-gamma-induced protein 10 (IP-10, also known as CXCL10).18 These molecules are central players in inflammatory cascades and serve as general indicators of ongoing immune activation.
The pro-inflammatory signature extends beyond these key cytokines. The same meta-analysis identified significant elevations in a broad array of mediators involved in myeloid cell activation and leukocyte trafficking, including IL-1β, IL-8 (CXCL8), Macrophage Inflammatory Protein-1-alpha (MIP-1α, or CCL3), CCL4, CCL5, and CXCL1.18 This pattern suggests a sustained activation of innate immune cells, such as monocytes and macrophages, as a key feature of the condition. Some of these markers, particularly elevated IL-6, have also been associated with severity during the acute phase of COVID-19, raising the possibility that they may hold prognostic value for predicting which individuals are at higher risk of developing PASC.20
The Cytokine Paradox: Hyperinflammation vs. Immune Exhaustion
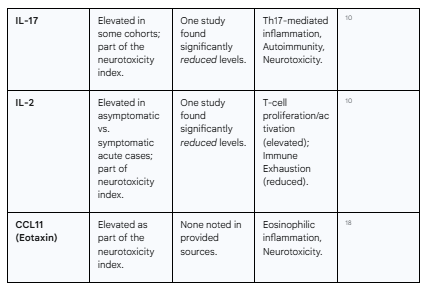
While the predominant narrative points to sustained hyperinflammation, this view is challenged by conflicting evidence that reveals a more nuanced and heterogeneous landscape. A notable study reported a dramatically different profile in its PASC cohort, finding 100% reductions in circulating Interferon-gamma (IFNγ) and IL-8, along with significant decreases in IL-6, IL-2, IL-17, IL-13, and IL-4 when compared to healthy individuals.10 The authors of this study proposed that their findings are indicative of a state of profound "immune exhaustion," where the immune system, after a prolonged period of activation, enters a state of functional hypo-responsiveness.10
This apparent paradox—some studies showing high levels of inflammatory cytokines while others show profound deficiencies—is a critical finding. It strongly suggests that PASC is not a single immunological entity. Rather, there are likely distinct immunological subtypes, or endotypes, that exist under the broad PASC umbrella. For instance, one patient subgroup may be characterized by a myeloid-driven, hyperinflammatory state with high IL-6 and TNF-α, consistent with the findings of the large meta-analysis. Another subgroup may be defined by T-cell exhaustion and a form of acquired immunodeficiency, characterized by low IFNγ and IL-2 production. This is further supported by a Brazilian cohort study which found elevated IL-17 and IL-2 in PASC patients, but, conversely, higher levels of the anti-inflammatory cytokine IL-10, along with IL-6 and IL-4, in individuals who had recovered from COVID-19 without developing persistent sequelae.21
This complexity means that a diagnostic panel cannot rely on a single marker. Instead, it must be a multiplex panel capable of discerning these distinct and sometimes opposing signatures. The diagnostic power may lie not in the absolute level of any single cytokine, but in the ratios and patterns among multiple mediators. For example, the ratio of a pro-inflammatory marker like TNF-α to a T-cell function marker like IFNγ could be a far more potent classifier of patient endotype than either analyte measured in isolation. This shifts the research objective from identifying a single "PASC cytokine" to defining "PASC cytokine patterns."
Signatures of Immune-Associated Neurotoxicity
One of the most debilitating aspects of PASC is the high prevalence of neurological and cognitive symptoms, including brain fog, memory impairment, headaches, and mood disturbances.2 Research has begun to uncover a specific biological basis for these symptoms, linking them to a distinct profile of immune mediators that exhibit neurotoxic properties. The large-scale meta-analysis aggregated several of these molecules into a composite "neurotoxicity index," which was found to be significantly elevated in PASC patients.18
This neurotoxic signature includes many of the pro-inflammatory cytokines and chemokines associated with general PASC inflammation, such as IL-1β, IL-6, and TNF-α, but also includes others like IL-16, IL-17, CCL2, CCL5, and CCL11 (Eotaxin).18 These molecules can cross a compromised blood-brain barrier or be produced intrathecally, where they can directly impair neuronal function, disrupt synaptic plasticity, promote neuroinflammation via microglial activation, and contribute to the pathophysiology of cognitive and affective symptoms.3 The identification of this specific signature provides a crucial mechanistic link between systemic immune dysregulation and the central nervous system manifestations of PASC.
The following table synthesizes the complex and sometimes contradictory findings regarding key cytokine and chemokine biomarkers in PASC, highlighting their association with distinct clinical phenotypes.
Table 1: Key Cytokine and Chemokine Signatures in PASC
Candidate Biomarkers: Autoantibody Profiles
The emergence of autoimmunity as a central driver of PASC represents one of the most significant advances in understanding the disease. The evidence has progressed from initial associations to direct demonstrations of causality, positioning autoantibody profiling as a cornerstone of any future diagnostic and stratification panel.
The Landscape of Humoral Autoimmunity in PASC
The profound immune perturbation caused by SARS-CoV-2 infection can disrupt self-tolerance, leading to the generation of antibodies that erroneously target the body's own tissues. Studies have shown that both severe and mild COVID-19 can induce a state of persistent humoral autoimmunity.27 A diverse array of autoantibodies has been detected in patients with PASC. This includes broad-acting autoantibodies, such as anti-nuclear antibodies (ANAs) and anti-extractable nuclear antigen (ENA) antibodies, which are commonly seen in systemic autoimmune diseases like lupus. The presence of ANAs in the months following acute infection has been shown to be predictive of subsequent PASC symptoms.13
Beyond these general markers of autoimmunity, more specific autoantibodies have been identified. These include autoantibodies directed against key functional proteins such as G-protein coupled receptors (GPCRs), which are critical for regulating autonomic functions, and components of the cardiovascular system like cardiolipin.9 The presence of these specific autoantibodies provides plausible mechanistic explanations for common PASC phenotypes such as autonomic dysfunction (dysautonomia), postural orthostatic tachycardia syndrome (POTS), and cardiovascular complications. Notably, patients with persistent neurologic and fatigue symptoms (a phenotype termed neuro-PASC) exhibit substantially higher and more diverse autoantibody responses compared to individuals who recover without sequelae, even after mild initial infections.27
Establishing Causality: From Patient Serum to Animal Models
A pivotal development in PASC research has been the transition from correlating autoantibodies with symptoms to demonstrating a direct causal role. Seminal experiments conducted by researchers at Yale and Mount Sinai involved purifying the IgG antibody fraction from the blood of PASC patients and healthy controls and transferring them into healthy mice.15 The results were striking: mice that received antibodies from PASC patients developed a range of behavioral changes that mirrored the symptoms of the human donors, whereas mice receiving control antibodies did not.16
The specificity of these findings provides powerful evidence for the pathogenic role of autoantibodies. For instance, 85% of mice that developed heightened sensitivity to a painful heat stimulus had received antibodies from patients who themselves reported pain as a major PASC symptom.15 Similarly, 89% of mice that struggled with a coordination and balance test had received antibodies from patients who reported dizziness.15 This remarkable one-to-one correlation suggests that distinct cocktails of autoantibodies present in different patients target specific tissues—such as peripheral nerves for pain or the inner ear or cerebellum for dizziness—to produce a corresponding clinical phenotype. These findings firmly establish that, for a significant portion of the PASC population, autoimmunity is not just an epiphenomenon but a direct driver of their disease.
High-Potential Diagnostic Autoantibodies: PITX2 and FBXO2
To move from broad observations to specific diagnostic tools, researchers have employed high-throughput screening methods to identify the precise molecular targets of these pathogenic autoantibodies. Using a comprehensive protein array containing approximately 20,000 human proteins, followed by rigorous validation with enzyme-linked immunosorbent assays (ELISA) in a large patient cohort, two novel and highly promising serum autoantibody biomarkers have been identified: antibodies targeting Paired-like homeodomain 2 (PITX2) and F-box protein 2 (FBXO2).28
The diagnostic performance of these autoantibodies was assessed using Receiver Operating Characteristic (ROC) curve analysis, which measures a test's ability to distinguish between two groups.
Anti-PITX2 Autoantibodies: This biomarker demonstrated high accuracy. It could distinguish PASC patients from COVID-19 convalescents without PASC with an Area Under the Curve (AUC) of 0.891, and from pre-pandemic healthy controls with an AUC of 0.866. An AUC of 1.0 represents a perfect test, while 0.5 represents a test with no discriminatory ability.28
Anti-FBXO2 Autoantibodies: This biomarker showed moderate but still significant accuracy, with an AUC of 0.762 when comparing PASC patients to non-PASC convalescents and an AUC of 0.786 against healthy controls.28
Furthermore, the levels of these autoantibodies correlated with specific clinical symptoms. Higher levels of anti-PITX2 were associated with reports of fever, palpitations, loss of appetite, and brain fog. This is mechanistically plausible, as PITX2 is a transcription factor known to be crucial for normal cardiac development and the function of neurons in brain regions involved in cognition.28 Higher levels of anti-FBXO2 were associated with dyspnea and loss of appetite. FBXO2 is a component of the ubiquitin-proteasome system, which is essential for maintaining protein homeostasis, and its disruption by autoantibodies could contribute to widespread cellular dysfunction and inflammation.28
The specificity observed in both the animal models and the identification of discrete autoantibody targets like PITX2 provides a clear path from a measurable biomarker to a specific clinical phenotype and a plausible biological mechanism. An autoantibody panel could therefore achieve more than a simple binary diagnosis of PASC. It could create a "mechanistic map" of an individual patient's disease. By measuring a panel of autoantibodies—including anti-PITX2, anti-FBXO2, anti-GPCRs, and others yet to be discovered—it may become possible to classify a patient not just as "PASC-positive," but as having a specific endotype, such as "cardiac/neuro-autoimmune PASC" or "dysautonomia-autoimmune PASC." This level of diagnostic precision has profound therapeutic implications. If a patient's symptoms are determined to be driven by pathogenic autoantibodies, then therapies specifically designed to remove or neutralize these antibodies—such as intravenous immunoglobulin (IVIg), plasmapheresis, or B-cell depleting agents—become the most logical and promising treatment strategies.15 This represents a direct and actionable application of personalized medicine.
Candidate Biomarkers: Cellular Immune Dysregulation
Beyond the soluble factors circulating in the blood, the cells of the immune system themselves undergo profound and lasting alterations in PASC. Detailed analysis of lymphocyte subsets, particularly T-cells and B-cells, reveals a landscape of chronic activation, functional exhaustion, and dysregulated memory formation that provides further clues to the underlying pathophysiology and offers a rich source of potential biomarkers.
T-Cell Compartment Alterations: The Exhausted Sentinels
The T-cell compartment, which is central to orchestrating the adaptive immune response and clearing viral infections, appears to be particularly affected in PASC. A cardinal feature observed months after the initial infection is a state of persistent T-cell activation coexisting with signs of functional exhaustion.30 This is evidenced by the sustained high expression of activation markers like HLA-DR, CD38, and CD69 on both CD4+ helper and CD8+ cytotoxic T-cells.30 Simultaneously, these cells show increased expression of negative immune checkpoint molecules, or "exhaustion markers," such as Programmed Cell Death Protein 1 (PD-1) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3).18 This dual signature of high activation and high exhaustion is a classic hallmark of an immune system engaged in a prolonged, unresolved battle against a persistent antigen.
This chronic stimulation drives a significant remodeling of the T-cell memory and effector subsets. One of the most consistent findings is a marked expansion of a population known as terminally differentiated effector memory RA+ (TEMRA) cells, especially within the CD8+ T-cell compartment.31 TEMRA cells are highly cytotoxic but have poor proliferative capacity and are often considered a marker of immunological aging or senescence. Their accumulation suggests a history of extensive antigenic stimulation. This expansion of effector cells comes at the cost of the naïve T-cell pool, which is significantly depleted in PASC patients.32 This depletion could reflect either impaired production of new T-cells from the thymus or the excessive differentiation of naïve cells into effector cells in response to the chronic immune trigger. In individuals recovering from severe COVID-19, this polarization towards a senescent state is particularly pronounced, with a high proportion of CD8+ T-cells expressing the senescence marker CD57.32
Functionally, the T-cell response in PASC also appears to be skewed. Several studies have noted a polarization away from a balanced Th1 response (critical for antiviral immunity) towards Th2 and Th17 responses.35 This shift is significant, as Th2 and Th17 cells are known to be key drivers of allergic and autoimmune diseases, respectively, lending further support to the autoimmunity hypothesis in PASC. Alterations have also been observed in circulating follicular helper T-cells (TFH), a specialized CD4+ T-cell subset that is essential for providing help to B-cells in germinal centers to produce high-affinity antibodies. A skewing of TFH populations could contribute to the production of poorly regulated or self-reactive antibodies.31
The diagnostic potential of these cellular markers is substantial. In one study, a machine learning model was trained on a wide array of immunological parameters to distinguish PASC patients from recovered controls. The model identified T-cell phenotypes, particularly the presence of terminally differentiated, SARS-CoV-2-specific CD4+ T-cells, as the most powerful predictive variables, achieving a classification accuracy of over 80%.34
B-Cell Abnormalities: Dysfunctional Antibody Factories
The B-cell compartment, responsible for producing antibodies, also shows signs of long-term dysregulation. During severe acute COVID-19, the B-cell response can be chaotic, characterized by derangements in germinal center reactions and an expansion of extrafollicular B-cell activation.4 This type of disorganized response is known to be prone to generating autoantibodies and may lay the groundwork for the autoimmune complications seen later in PASC.
Following the acute phase, a key finding, particularly in those who recovered from critical illness, is the development of a persistent B-cell lymphopenia, defined by a low count of circulating CD19+ B-cells.37 This B-cell deficiency has been observed to last for at least 50 days and, in some studies, for as long as eight months after the initial infection.37 This suggests a long-lasting impairment in the body's ability to produce or maintain its B-cell population, which could have implications for long-term immunity and the regulation of antibody responses.
The specific pattern of T-cell dysregulation observed in PASC provides a powerful, non-invasive window into one of the core mechanistic pillars of the disease: viral persistence. The triad of high T-cell activation, profound T-cell exhaustion, and a skewing of the memory compartment towards senescent TEMRA cells is the quintessential immunological signature of a chronic, unresolved viral infection. While directly proving the existence of viral reservoirs in tissues like the gut or brain requires invasive biopsies, which are impractical for routine clinical diagnosis, the immune system's response offers a proxy measurement.38 T-cells are exquisitely designed to respond to viral antigens. A persistent source of these antigens, as hypothesized in the viral reservoir theory, would inevitably lead to the exact T-cell phenotype seen in PASC: continuous activation (due to antigen presence), subsequent exhaustion (as the response fails to clear the source), and accelerated differentiation (depleting the naïve pool and accumulating TEMRA cells). The strong predictive power of T-cell features in machine learning models validates this concept.34 Therefore, a multi-parameter flow cytometry panel designed to quantify these specific T-cell subsets and their expression of activation and exhaustion markers could serve as a highly specific surrogate biomarker for underlying viral persistence, potentially guiding the use of antiviral therapies without the need for invasive procedures.
From Biomarkers to Clinical Utility: Patient Stratification and Immunological Endotypes
The ultimate goal of biomarker research in PASC is not merely to diagnose the condition but to deconstruct its clinical heterogeneity into biologically meaningful and therapeutically actionable subgroups. By integrating the diverse biomarker data from cytokine profiles, autoantibody screens, and cellular analyses, it is possible to move beyond broad, symptom-based phenotypes toward the definition of distinct immunological endotypes, each driven by a specific pathophysiological mechanism.
The Imperative for Stratification: Moving Beyond the "Long COVID" Monolith
PASC is fundamentally not a single disease. As an umbrella term for a condition with over 200 symptoms affecting virtually every organ system, it is a clear example of a heterogeneous syndrome.2 Current definitions from major health organizations like the CDC, WHO, and the National Academies of Sciences, Engineering, and Medicine (NASEM) are necessarily broad, relying on symptom duration and history of infection rather than objective biological criteria.40 While useful for public health surveillance, this lack of biological definition creates a "moving target" for research and clinical trials, as different studies may inadvertently enroll vastly different patient populations under the same "Long COVID" label.42
To advance the field, a shift from phenotype to endotype is required. A phenotype is an observable characteristic or symptom cluster (e.g., "patients with fatigue and brain fog"). An endotype, in contrast, is a subtype of a condition defined by a distinct underlying functional or pathobiological mechanism.44 Identifying these endotypes is the crucial step that enables the development of targeted, personalized therapies.
Proteomic and Symptom-Based Phenotypes
The first step toward defining endotypes is to establish that subjective symptom clusters have objective biological correlates. Large-scale studies analyzing hundreds of plasma proteins in PASC patients have successfully achieved this, identifying distinct inflammatory signatures that are associated with specific symptom groups.46 This work provides a crucial bridge between patient experience and underlying biology. Key associations include:
Cardiorespiratory, Fatigue, and Anxiety/Depression Phenotype: Associated with elevated plasma levels of IL-1 Receptor Type 2 (IL-1R2), Matrilin-2 (MATN2), and Collectin-12 (COLEC12).46
Gastrointestinal Phenotype: Associated with elevated MATN2, Colony Stimulating Factor 3 (CSF3), Complement C1q A Chain (C1QA), and Secretogranin-3 (SCG3), a marker suggestive of disturbances in the brain-gut axis.46
Cognitive Impairment ("Brain Fog") Phenotype: Associated with elevated C1QA and markers of ongoing nerve tissue repair or damage, such as Spondin-1 (SPON-1) and Neurofascin (NFASC).46
These findings validate the clinical reality of different PASC presentations and provide a proteomic foundation upon which more detailed immunological endotypes can be built.
Proposed Immunological Endotypes of PASC
By synthesizing the full spectrum of biomarker evidence—from soluble mediators to autoantibodies to cellular profiles—it is possible to propose several core immunological endotypes that likely drive the observed clinical phenotypes. It is important to note that these endotypes are not necessarily mutually exclusive, and an individual patient may exhibit features of more than one.
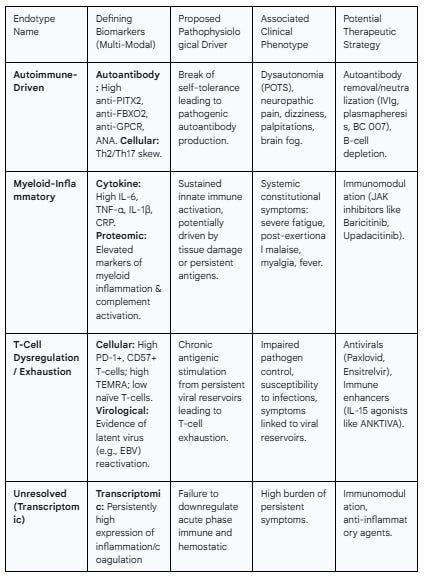
Endotype 1: The "Autoimmune-Driven" Endotype
Defining Biomarkers: The hallmark of this endotype is the presence of high titers of specific, pathogenic autoantibodies. This includes newly identified markers like anti-PITX2 and anti-FBXO2, as well as previously described autoantibodies against GPCRs and other tissue-specific antigens.28 Broader markers like ANAs may also be present.13 The cellular profile may show a T-cell polarization towards Th2 and/or Th17 responses, which are known to support autoantibody production.35
Associated Clinical Phenotype: This endotype is strongly associated with autonomic dysfunction, including POTS and orthostatic intolerance, as well as neuropathic pain, dizziness, palpitations, and other symptoms that correlate directly with the identified autoantibody target.9 Epidemiological data suggest this endotype may be more prevalent in women.15
Endotype 2: The "Myeloid-Inflammatory" Endotype
Defining Biomarkers: This endotype is characterized by evidence of sustained activation of the innate immune system, particularly myeloid cells (monocytes/macrophages). Key biomarkers include elevated plasma markers of myeloid inflammation and complement activation 46, high circulating levels of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β, and elevated acute-phase reactants like C-reactive protein (CRP).18
Associated Clinical Phenotype: This endotype likely underlies the more systemic, constitutional symptoms of PASC, including severe fatigue, post-exertional malaise, myalgia (muscle pain), and low-grade fevers. It is also linked to the cardiorespiratory symptom cluster.6
Endotype 3: The "T-Cell Dysregulation/Exhaustion" Endotype
Defining Biomarkers: This endotype is defined by a distinct signature in the T-cell compartment. This includes a high frequency of exhausted (PD-1+) and senescent (CD57+) CD8+ T-cells, an expanded population of CD8+ TEMRA cells, and a contracted naïve T-cell pool.31 This profile may be accompanied by laboratory evidence of latent virus reactivation, such as circulating EBV DNA or high EBV antibody titers.5
Associated Clinical Phenotype: The clinical presentation may be linked to symptoms driven by persistent, low-level viral reservoirs. Functionally, this endotype may also confer a state of relative immunodeficiency, leading to an increased susceptibility to secondary or opportunistic infections.
Transcriptomic Endotypes: A Systems-Level View
Complementing the classification based on protein and cellular biomarkers, a systems-level approach using whole blood transcriptomics (gene expression profiling) has identified endotypes based on the longitudinal trajectory of a patient's immune and hemostatic response. This powerful approach captures the dynamic nature of the immune system's (mal)adaptation over time.6
"Resolved" Endotype: This group represents the trajectory of successful recovery and has the lowest rate of persistent post-COVID symptoms. It is characterized by a robust and appropriate inflammatory and coagulation response during the acute hospitalized phase, which then successfully de-escalates and returns to baseline after discharge.
"Suppressive" Endotype: This group has a very high rate of post-COVID symptoms (85.7%). Its trajectory is defined by a persistently dampened or suppressed inflammatory and coagulation response, both during and after hospitalization. This endotype can be identified by a specific 6-gene signature that includes IGKV1-27 and CEACAM19.6
"Unresolved" Endotype: This group also has a high rate of post-COVID symptoms (40%). Its trajectory is the opposite of the "Suppressive" endotype, defined by a persistently activated and unresolved state of inflammation and coagulation that fails to return to baseline after discharge. This endotype is defined by a distinct 6-gene signature that includes PCSK9 and C4BPA.6
The following table provides a synthesized framework of the proposed immunological endotypes of PASC, linking their defining biomarkers to underlying pathophysiology, clinical presentation, and potential therapeutic avenues.
Table 2: Proposed Immunological Endotypes of PASC
Prognostic Value and Biomarker-Guided Therapeutic Strategies
The development of a robust biomarker panel for PASC extends beyond diagnosis and stratification; its ultimate clinical value lies in its ability to predict disease course and, most importantly, to guide the selection of targeted, personalized therapies. By matching specific treatments to the underlying biological mechanisms driving a patient's illness, it becomes possible to move from supportive care to precision medicine.
Predicting Disease Course and Severity
Biomarkers measured during the acute phase of COVID-19 or in the early convalescent period may hold significant prognostic value for identifying individuals at high risk of developing PASC. Studies have shown that elevated levels of markers of inflammation and coagulopathy, such as D-dimer, lactate dehydrogenase (LDH), IL-6, and IP-10, as well as lymphopenia (low lymphocyte count) during the acute illness, are associated with more severe disease, higher mortality, and may predict a higher likelihood of subsequent long-term sequelae.22
In the post-acute phase, the presence of certain biomarkers can predict the persistence of symptoms. For example, the detection of anti-nuclear autoantibodies (ANAs) two to three months after the initial infection is predictive of ongoing PASC symptoms.27 Furthermore, the transcriptomic endotypes identified through gene expression profiling have direct prognostic implications. Patients whose immune and hemostatic responses follow the "Suppressive" or "Unresolved" trajectories are significantly more likely to experience a high burden of persistent symptoms compared to those in the "Resolved" endotype.6 These gene signatures could potentially be developed into prognostic tests to identify high-risk patients early and allocate resources or preventative interventions accordingly.
A Framework for Personalized, Biomarker-Guided Medicine
The identification of distinct immunological endotypes provides a rational framework for designing and implementing personalized therapeutic strategies. Instead of treating "Long COVID" as a single entity, clinicians can target the specific pathological pathway active in a given patient, as identified by their biomarker profile.
Targeting the "Myeloid-Inflammatory" Endotype: For patients whose PASC is characterized by high levels of pro-inflammatory cytokines like IL-6 and TNF-α, the most logical approach is immunomodulation. Janus kinase (JAK) inhibitors are a class of oral medications that block the intracellular signaling pathways used by many of these cytokines. Several large-scale clinical trials are currently underway to test this hypothesis. The REVERSE-LC trial is a Phase 3 study evaluating the efficacy of baricitinib (Olumiant), a JAK1/2 inhibitor, in improving neurocognitive and physical function in PASC.51 Similarly, the international LC-REVITALIZE platform trial is investigating
upadacitinib (Rinvoq), a selective JAK1 inhibitor.53 Success in these trials would validate the "Myeloid-Inflammatory" endotype and provide a targeted therapy for this patient subgroup.Targeting the "Autoimmune-Driven" Endotype: In patients where pathogenic autoantibodies are the primary driver of disease, therapies should focus on removing, neutralizing, or halting the production of these antibodies. Intravenous immunoglobulin (IVIg), a product derived from pooled human plasma containing a wide array of antibodies, is thought to work by neutralizing pathogenic autoantibodies and providing other immunomodulatory effects. The RECOVER-AUTONOMIC trial is specifically testing IVIg in PASC patients who have developed POTS, a condition strongly linked to autoimmunity.54 Other potential approaches include plasmapheresis (a procedure to physically remove antibodies from the blood) and novel agents like BC 007, a DNA-based aptamer designed to neutralize pathogenic autoantibodies directed against GPCRs.15
Targeting the "T-Cell Dysregulation/Exhaustion" Endotype (and Viral Persistence): This endotype, which is strongly suggestive of an underlying persistent viral reservoir, calls for a dual therapeutic strategy.
Antiviral Therapy: The primary goal is to eliminate the source of chronic antigenic stimulation. Antiviral drugs such as Paxlovid (nirmatrelvir/ritonavir) and ensitrelvir are being tested in clinical trials to determine if they can clear persistent SARS-CoV-2 and thereby allow the immune system to reset and symptoms to resolve.56
Immune Enhancement: A complementary approach is to directly boost the function of the exhausted T-cells and Natural Killer (NK) cells. ANKTIVA (nogapendekin alfa inbakicept-pmln) is an IL-15 superagonist designed to stimulate the proliferation and activity of these critical antiviral lymphocytes. A Phase 2 clinical trial is currently evaluating ANKTIVA's ability to improve NK and CD8+ T-cell counts and function in PASC patients.59
The evolution of clinical trial design for PASC provides a powerful lesson in the importance of a biomarker-driven approach. Early trials that tested therapies like Paxlovid in a broad, unstratified PASC population failed to show significant benefits.56 This negative result does not necessarily mean the drug is ineffective for PASC; rather, it likely reflects the fact that it was administered to a heterogeneous population where only a fraction of patients—perhaps the 25-35% estimated to have evidence of viral persistence—had the relevant underlying pathology.56 Giving an antiviral to a patient whose symptoms are driven purely by autoimmunity is unlikely to be effective and will dilute any potential positive signal from the appropriate subgroup, leading to an overall negative trial result. In response to these early failures, newer trials are incorporating biomarkers into their design. The RECOVER-VITAL trial, for example, is using a highly sensitive blood test for viral proteins to prospectively identify patients with evidence of viral persistence and will measure whether antiviral treatment successfully clears these proteins.60 This represents a critical paradigm shift: the goal is no longer to treat "Long COVID" but to treat specific, biologically defined endotypes like "Autoimmune PASC," "Inflammatory PASC," or "Viral Persistence PASC." The future of effective PASC therapeutics is therefore inextricably linked to the successful development and implementation of these biomarker panels.
Challenges and Future Directions in PASC Biomarker Research
While the path toward a clinically actionable biomarker panel for PASC is clear and promising, several significant challenges must be addressed. Overcoming these hurdles will require a concerted, collaborative, and well-funded research effort. The successful development of such a panel would not only revolutionize the care of PASC patients but also establish a new paradigm for diagnosing and managing other complex, infection-associated chronic illnesses.
Overcoming Hurdles in Development and Validation
The journey from a promising research finding to a validated clinical diagnostic test is fraught with challenges, many of which are amplified in the context of a novel and heterogeneous condition like PASC.
The Definitional Challenge: A primary obstacle is the lack of a single, universally accepted, biologically-grounded definition for PASC. Current definitions are based on symptom duration, leading to the inclusion of highly heterogeneous patient populations in research cohorts.42 This creates a "moving target" for biomarker validation; a marker that performs well in a cohort defined by one set of criteria may perform poorly in another, making it difficult to compare results across studies and establish generalizability.61
The "Gold Standard" Problem: The validation of any new diagnostic test requires comparison against an established "gold standard" to accurately determine its sensitivity and specificity.62 For PASC, no such gold standard exists; the diagnosis is currently one of clinical judgment based on symptoms and exclusion of other conditions.43 This creates a circular dilemma: it is difficult to validate a biological marker against a non-biological definition. Progress will likely require an iterative process where initial biomarker candidates help to refine the disease definition, which in turn allows for better validation of the next generation of biomarkers.
Technical and Logistical Challenges: Several practical hurdles also stand in the way.
Funding: Despite the massive public health burden of PASC, research has been historically underfunded, slowing the pace of discovery and validation.38
Sample Accessibility: Some of the most definitive evidence for PASC pathology, such as proving viral persistence in tissues, requires invasive procedures like gut biopsies, which are not scalable or suitable for routine clinical diagnostics.38 This reinforces the importance of developing robust blood-based proxy markers.
Validation Rigor: A candidate biomarker must be rigorously validated in large, independent, and diverse patient cohorts to ensure its performance is robust across different populations, ethnicities, and geographic locations. This is a time-consuming and expensive process but is absolutely essential for clinical translation.38 Large-scale, coordinated efforts like the NIH's RECOVER initiative are designed to provide the necessary infrastructure and patient numbers to perform this critical validation work.64
The Path to a Clinically Actionable Panel
Despite the challenges, a clear roadmap for developing and implementing a PASC biomarker panel is emerging. This path requires a multi-pronged, systems-level approach that integrates cutting-edge science with pragmatic clinical development.
A Multi-Omic, Systems Biology Approach: The complexity of PASC demands a research strategy that moves beyond single-analyte studies. The most promising path forward, as articulated by the RECOVER OMICS task force, involves the simultaneous and longitudinal analysis of multiple data layers—including proteomics (proteins), transcriptomics (gene expression), immunophenotyping (cell types), and metabolomics (metabolites)—from the same patients. By integrating these multi-omic datasets with deep clinical phenotyping data, powerful machine learning algorithms can identify the most robust and predictive biomarker signatures that define distinct disease subtypes.64
Defining Statistical Rigor: For a biomarker panel to be clinically useful, it must meet stringent statistical criteria for diagnostic accuracy. This includes demonstrating high sensitivity (the ability to correctly identify patients who have PASC, minimizing false negatives) and high specificity (the ability to correctly identify individuals who do not have PASC, minimizing false positives).62 The trade-off between these two metrics must be carefully considered depending on the intended use of the test (e.g., a screening test may prioritize sensitivity, while a confirmatory test may prioritize specificity).63 The panel's positive and negative predictive values (PPV and NPV), which are influenced by disease prevalence, must also be established.62
Roadmap for Implementation: A phased approach is required to move from research discovery to clinical practice:
Discovery: Continue to support large-scale, multi-omic longitudinal studies like RECOVER to identify and refine candidate biomarkers and signatures for the various PASC endotypes.
Validation: Systematically validate the most promising signatures in large, independent, and international cohorts to confirm their accuracy and generalizability.
Assay Development: Partner with diagnostic companies to translate the validated biomarker signatures into standardized, high-throughput, and cost-effective clinical assays that can be performed in routine clinical laboratories (e.g., CLIA-certified multiplex panels).67
Biomarker-Stratified Clinical Trials: Design and execute the next generation of therapeutic trials for PASC that use the validated biomarker panel to enroll patients into specific treatment arms based on their immunological endotype. Proving that this biomarker-guided approach leads to improved patient outcomes is the ultimate validation of the panel's clinical utility.
Clinical Integration and Education: Develop clear clinical practice guidelines for the use and interpretation of the biomarker panel and disseminate this knowledge to frontline healthcare providers to ensure its proper integration into patient care.42
Concluding Remarks: A Paradigm Shift in Post-Viral Illness
The development of a robust, multi-modal immune biomarker panel for PASC is not only a scientifically feasible goal but represents the most critical unmet need in the field. Such a panel is the key to transforming PASC from a poorly understood, symptom-defined syndrome into a collection of well-defined, biologically-based diseases with targeted and effective treatments. The research to date has laid a remarkable foundation, identifying numerous high-potential candidates across the cytokine, autoantibody, and cellular domains and, most importantly, revealing the underlying immunological endotypes that drive this heterogeneous condition.
The successful implementation of such a panel will have profound implications. For the millions of individuals suffering from PASC, it offers the promise of an objective diagnosis, a clearer prognosis, and access to personalized therapies that target the root cause of their illness. For the scientific and medical communities, the effort to unravel PASC will create a new paradigm for the investigation, diagnosis, and treatment of other complex, infection-associated chronic conditions, including ME/CFS and post-Lyme disease syndrome. The knowledge, tools, and strategies forged in response to the challenge of Long COVID will undoubtedly have a lasting and positive impact on medicine for decades to come.
Acknowledgement
I acknowledge the use of Gemini AI in the preparation of this report. Specifically, it was used to: (1) brainstorm and refine the initial research questions; (2) assist in writing and debugging Python scripts for statistical analysis; and (3) help draft, paraphrase, and proofread sections of the final manuscript. I reviewed, edited, and assume full responsibility for all content.
Works cited
Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response - MDPI, accessed September 11, 2025, https://www.mdpi.com/2075-1729/13/11/2121
Pathogenic mechanisms of post- acute sequelae of SARS- CoV- 2 infection (PASC) - ScienceOpen, accessed September 11, 2025, https://www.scienceopen.com/document_file/c785ac41-18d6-45a3-8992-48cf38332950/PubMedCentral/c785ac41-18d6-45a3-8992-48cf38332950.pdf
Mechanistic Insights Into Long Covid: Viral Persistence, Immune Dysregulation, and Multi-Organ Dysfunction - PubMed, accessed September 11, 2025, https://pubmed.ncbi.nlm.nih.gov/40474772/
Immune mechanisms underlying COVID-19 pathology and post ..., accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10219649/
Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC) - eLife, accessed September 11, 2025, https://elifesciences.org/articles/86002
Post-COVID symptoms are associated with endotypes reflecting ..., accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10482103/
Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC) | eLife, accessed September 11, 2025, https://elifesciences.org/articles/86014
Mechanisms of long COVID: An updated review - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11332859/
Autoimmunity in Long Covid and POTS - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10224806/
Cytokine deficiencies in patients with Long-COVID - Longdom Publishing, accessed September 11, 2025, https://www.longdom.org/open-access/cytokine-deficiencies-in-patients-with-longcovid-95498.html
Pathophysiological, immunological, and inflammatory features of long COVID - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10932978/
Long Covid: Untangling the Complex Syndrome and the Search for Therapeutics - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9864843/
Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms - ERS Publications, accessed September 11, 2025, https://publications.ersnet.org/highwire_display/entity_view/node/597302/full
Autoantibodies in COVID-19 survivors with post-COVID symptoms: a systematic review, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1428645/full
Antibodies From Long COVID Patients Provide Clues to ..., accessed September 11, 2025, https://www.yalemedicine.org/news/antibodies-from-long-covid-patients-provide-clues-to-autoimmunity-hypothesis
New Evidence Supports Autoimmunity as One of Long COVID's Underlying Drivers, accessed September 11, 2025, https://medicine.yale.edu/news-article/new-evidence-supports-autoimmunity-as-one-of-long-covids-underlying-drivers/
IgA autoimmunity and coagulation among post-acute sequelae of SARS-CoV-2 infection (PASC) patients with persistent respiratory symptoms: a case-control study - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1589559/full
Immune activation and immune-associated neurotoxicity in Long ..., accessed September 11, 2025, https://www.medrxiv.org/content/10.1101/2024.02.08.24302516v1.full-text
Long COVID and autoimmunity: the role of cytokines - Cell Guidance Systems, accessed September 11, 2025, https://www.cellgs.com/blog/long-covid-and-autoimmunity-the-role-of-cytokines.html
COVID-19 disease and immune dysregulation - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9568269/
Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome, accessed September 11, 2025, https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2022.922422/full
Prognostic Value of Biomarkers in COVID-19: Associations with Disease Severity, Viral Variants, and Comorbidities—A Retrospective Observational Single-Center Cohort Study - MDPI, accessed September 11, 2025, https://www.mdpi.com/2075-1729/15/4/634
Utility of laboratory and immune biomarkers in predicting disease progression and mortality among patients with moderate to severe COVID-19 disease at a Philippine tertiary hospital - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1123497/full
Biomarkers in long COVID-19: A systematic review - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9895110/
Biomarkers in long COVID-19: A systematic review - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1085988/full
Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles - MDPI, accessed September 11, 2025, https://www.mdpi.com/2218-273X/15/7/928
Mild Primary or Breakthrough SARS-CoV-2 Infection Promotes Autoantibody Production in Individuals with and without Neuro-PASC | ImmunoHorizons | Oxford Academic, accessed September 11, 2025, https://academic.oup.com/immunohorizons/article/8/8/577/7848617
Identification of Putative Serum Autoantibodies Associated with Post ..., accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11855120/
Screening for autoantibodies in PASC serum using protein bead arrays.... - ResearchGate, accessed September 11, 2025, https://www.researchgate.net/figure/Screening-for-autoantibodies-in-PASC-serum-using-protein-bead-arrays-A-Representative_fig2_389151554
Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10319436/
T cell perturbations persist for at least 6 months following hospitalization for COVID-19, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.931039/full
Remodeling of T Cell Dynamics During Long COVID Is ... - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.886431/full
Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months | eLife, accessed September 11, 2025, https://elifesciences.org/articles/85009
Immunological and Clinical Markers of Post‐acute Sequelae of COVID‐19: Insights from Mild and Severe Cases 6 Months Post‐infection, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12238838/
T Cell Dynamics in COVID-19, Long COVID and Successful Recovery - ResearchGate, accessed September 11, 2025, https://www.researchgate.net/publication/394060033_T_Cell_Dynamics_in_COVID-19_Long_COVID_and_Successful_Recovery
T Cell Dynamics in COVID-19, Long COVID and Successful Recovery - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12346483/
Persistent CD19+ B cell lymphopenia in critically ill COVID-19 patients 50 days after symptom onset - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2024.1488607/full
Finding an accepted Long COVID biomarker will be complicated. This blood test could give us answers. - The Sick Times, accessed September 11, 2025, https://thesicktimes.org/2025/01/21/finding-an-accepted-long-covid-biomarker-will-be-complicated-this-blood-test-could-give-us-answers/
What is long COVID? | American Medical Association, accessed September 11, 2025, https://www.ama-assn.org/public-health/infectious-diseases/what-long-covid
Long COVID Basics - Centers for Disease Control and Prevention | CDC, accessed September 11, 2025, https://www.cdc.gov/long-covid/about/index.html
Examining the Working Definition for Long COVID | National Academies, accessed September 11, 2025, https://www.nationalacademies.org/our-work/examining-the-working-definition-for-long-covid
Long COVID: General Perceptions and Challenges in Diagnosis and Management - MDPI, accessed September 11, 2025, https://www.mdpi.com/2673-8112/5/3/41
Challenges in diagnosis and treatment of long COVID - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2025.1641411/full
Identification of Endotypes of Hospitalized COVID-19 Patients - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8632028/
Pan-vaccine analysis reveals innate immune endotypes predictive of antibody responses to vaccination. - Apollo - University of Cambridge, accessed September 11, 2025, https://www.repository.cam.ac.uk/items/a6379506-95e4-4a16-8e07-1002b67558f4
Large-scale phenotyping of patients with long COVID post ..., accessed September 11, 2025, https://researchportal.ukhsa.gov.uk/en/publications/large-scale-phenotyping-of-patients-with-long-covid-post-hospital
Blood Biomarkers Reveal the Hidden World of Long COVID - SciTechDaily, accessed September 11, 2025, https://scitechdaily.com/blood-biomarkers-reveal-the-hidden-world-of-long-covid/
Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10341908/
Molecular and Clinical Prognostic Biomarkers of COVID-19 Severity and Persistence - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8948624/
Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19 - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.857573/full
Treatment on Trial to Reverse Long COVID Effects - Discoveries in Medicine, accessed September 11, 2025, https://discoveries.vanderbilthealth.com/2025/01/treatment-on-trial-to-reverse-long-covid-effects/
NIH-funded trial to determine if immunomodulation can improve brain and cardiovascular dysfunction in Long COVID - VUMC News, accessed September 11, 2025, https://news.vumc.org/2024/02/22/nih-funded-trial-to-determine-if-immunomodulation-can-improve-brain-and-cardiovascular-dysfunction-in-long-covid/
Three clinical trials for Long COVID are testing JAK inhibitors to treat immune dysregulation, accessed September 11, 2025, https://thesicktimes.org/2025/08/05/three-clinical-trials-for-long-covid-are-testing-jak-inhibitors-to-treat-immune-dysregulation/
NIH Launches Trials for Long COVID Treatment Breakthroughs, accessed September 11, 2025, https://www.autoimmuneinstitute.org/covid_timeline/nih-launches-trials-for-long-covid-treatment-breakthroughs/
The current landscape of long COVID clinical trials: NIH's RECOVER to Stanford Medicine's STOP-PASC initiative - PMC, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10481149/
Early Long COVID Trials of Paxlovid and Monoclonal Antibodies Show No Significant Benefits, But Research Continues - MedPath, accessed September 11, 2025, https://trial.medpath.com/news/24fc9eec5d1e1daa/early-long-covid-trials-of-paxlovid-and-monoclonal-antibodies-show-no-significant-benefits-but-research-continues
Ensitrelvir for Viral Persistence and Inflammation in People Experiencing Long COVID - UCSF Clinical Trials, accessed September 11, 2025, https://clinicaltrials.ucsf.edu/trial/NCT06161688
Stanford Medicine clinical trial goals: Meet long COVID head-on, treat it and defeat it, accessed September 11, 2025, https://med.stanford.edu/news/all-news/2023/03/long-covid-pandemic-anniversary.html
ImmunityBio Announces Phase 2 Study of ANKTIVA® in Patients with Long COVID, accessed September 11, 2025, https://immunitybio.com/immunitybio-announces-phase-2-study-of-anktiva-in-patients-with-long-covid/
Study Finds Persistent Infection Could Explain Long COVID in Some People, accessed September 11, 2025, https://www.massgeneralbrigham.org/en/about/newsroom/press-releases/study-finds-persistent-infection-could-explain-long-covid-in-some-people
Challenges and Opportunities in Long COVID Research - PMC - PubMed Central, accessed September 11, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11210969/
Diagnostic Testing Accuracy: Sensitivity, Specificity, Predictive Values and Likelihood Ratios - StatPearls - NCBI Bookshelf, accessed September 11, 2025, https://www.ncbi.nlm.nih.gov/books/NBK557491/
Methods for Evaluating the Accuracy of Diagnostic Tests - CPP : Cardiovascular Prevention and Pharmacotherapy, accessed September 11, 2025, https://www.e-jcpp.org/journal/view.php?doi=10.36011/cpp.2021.3.e2
A multi-omics strategy to understand PASC through the RECOVER cohorts: a paradigm for a systems biology approach to the study of chronic conditions - Frontiers, accessed September 11, 2025, https://www.frontiersin.org/journals/systems-biology/articles/10.3389/fsysb.2024.1422384/epub
R3 Seminar Recap: Characterization of PASC and investigation of biomarkers: Insights from the RECOVER adult cohort, accessed September 11, 2025, https://recovercovid.org/r3-seminar-series/characterization-pasc-and-investigation-biomarkers-insights-recover-adult-cohort
Factsheet: Understanding the Accuracy of Diagnostic and Serology Tests: Sensitivity and Specificity - Johns Hopkins Center for Health Security, accessed September 11, 2025, https://centerforhealthsecurity.org/sites/default/files/2022-11/201207-sensitivity-specificty-factsheet.pdf
Long COVID: Advanced Diagnostic Insights - Eve Technologies, accessed September 11, 2025, https://www.evetechnologies.com/long-covid-diagnostic-insights/